Module 13: Citric Acid Cycle
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
35 Terms
Citric Acid Cycle
Condensation of Acetyl-CoA group (2 carbons) to Oxaloacetate (CoA comes off Acetyl-CoA)
This forms Citrate
3 reactions reduce NAD+ to NADH ~ 2.5 ATP
1 reaction reduces FAD+ to FADH2 ~ 1.5 ATP
two carbons are removed in the form of CO2
A GTP is also produced
What must happen to pyruvate before entering TCA cycle?
must be converted to Acetyl-CoA before entering TCA
Fate of pyruvate during TCA cycle...
Pyruvate is completely oxidized (CO2 waste)
oxidatively decarboxylated by pyruvate dehydrogenase, releasing CO2
reduces NAD+ to NADH because it is oxidized
What happened to the remaining carbon fragments in pyruvate?
the remaining two carbon fragment is covalently bonded to Coenzyme A
Fate of hydrogen during TCA cycle
- the hydrogens with their high-energy electrons ultimately react with O2 to generate H2O
Decarboxylation of pyruvate to generate acetyl CoA
- occurs in the matrix of mitochondria
- Oxidation of pyruvate and transfer of high energy electrons to NAD+ to form NADH
- One of the Carbons in Actyl-CoA is converted to CO2 as a product of this reaction
- There is a large -∆G associated with this reaction and so it is also irreversible.
Acetyl CoA
very important intermediate in energy metabolism
regulator: regulated by the activity of pyruvate dehydrogenase
Pyruvate dehydrogenase
A multienzyme complex
increased reaction rate due to frequent substrate collision with multiple reactions happening
reduced probability of competing reactions
coordinated control
The coenzymes required for these reactions include thiamine pyrophosphate (TPP), lipoic acid, CoA, FAD and NAD+
Reaction 1: decarboxylation of pyruvate
Reactant: Pyruvate
Enzyme: Pyruvate dehydrogenase
Cofactor: Thiamine pyrophosphate (TPP) —> decarboxylates pyruvate
Product: hydroxyethyl TPP carbanion (intermediate)
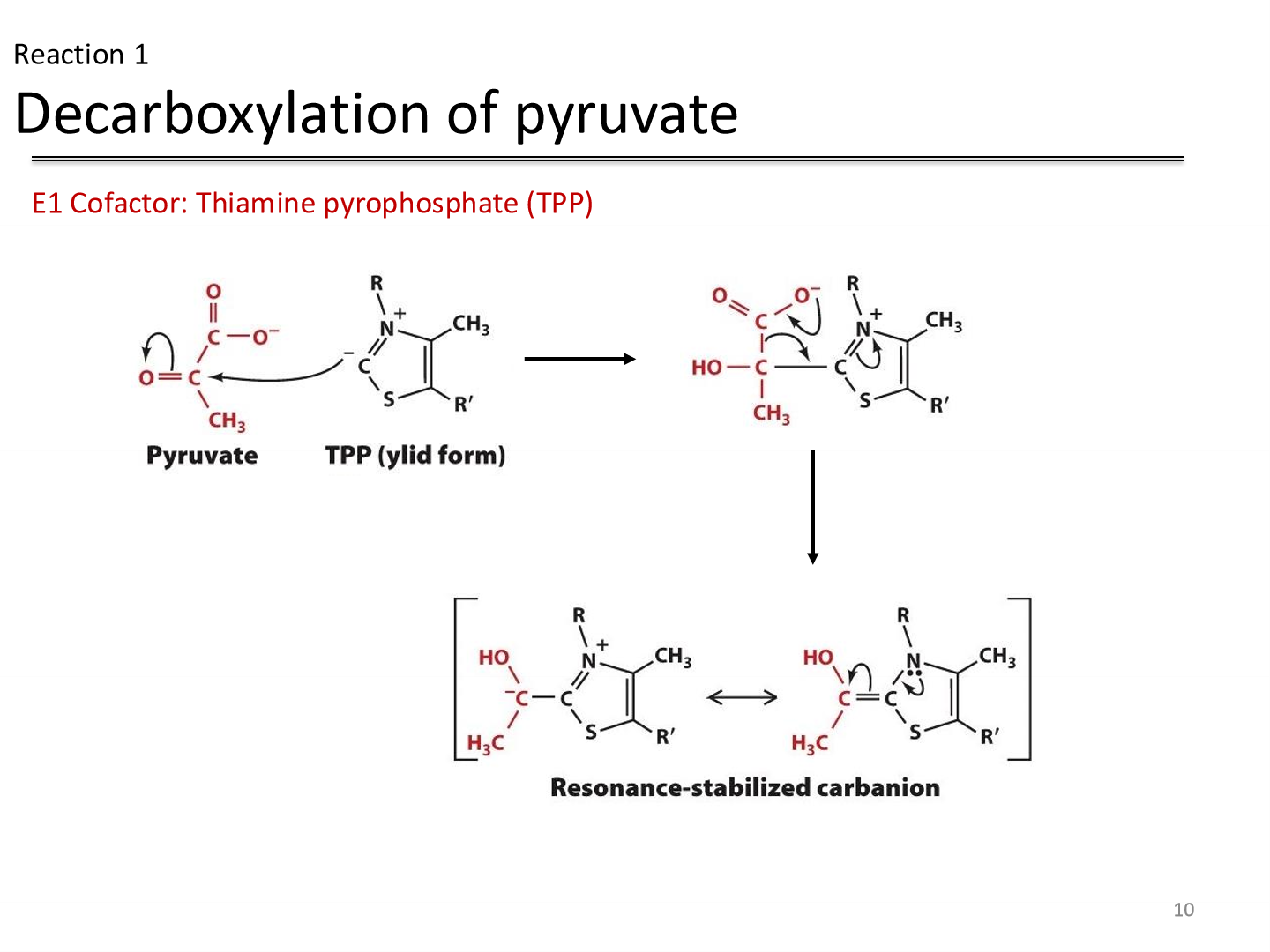
Thiamine pyrophosphate (TPP)
coenzyme most utilized in alpha-keto acid decarboxylation reactions because of the ability of its thiazolium ring to add to carbonyl groups
has an acidic proton that can be readily deprotonated
Pyruvate dehydrogenase regulation by product inhibition
NADH and acetyl-CoA
High NADH and acetyl-CoA ratios maintain E2 in the acetylated form, incapable of accepting the hydroxyethyl group from the TPP on E1
this decreases the rate of pyruvate decarboxylation
Pyruvate dehydrogenase regulation by covalent modification
phosphorylation/dephosphorylation of E1
in response to increases in blood glucose, insulin promotes the synthesis of acetyl-CoA
insulin and Ca2+activates pyruvate dehydrogenase phosphatase, which removes the phosphate groups from pyruvate dehydrogenase
Reaction 2: Acetylation of Lipoamide
Reactant: hydroxyethyl TPP carbanion —> attacks the Lipoamide disulfide
Enzyme: Dihydrolipoyl transacetylase
Cofactor: Lipoic acid, covalently linked to a Lys on the enzyme
Product: TPP is eliminated and acetyl-dihydrolipoamide remains
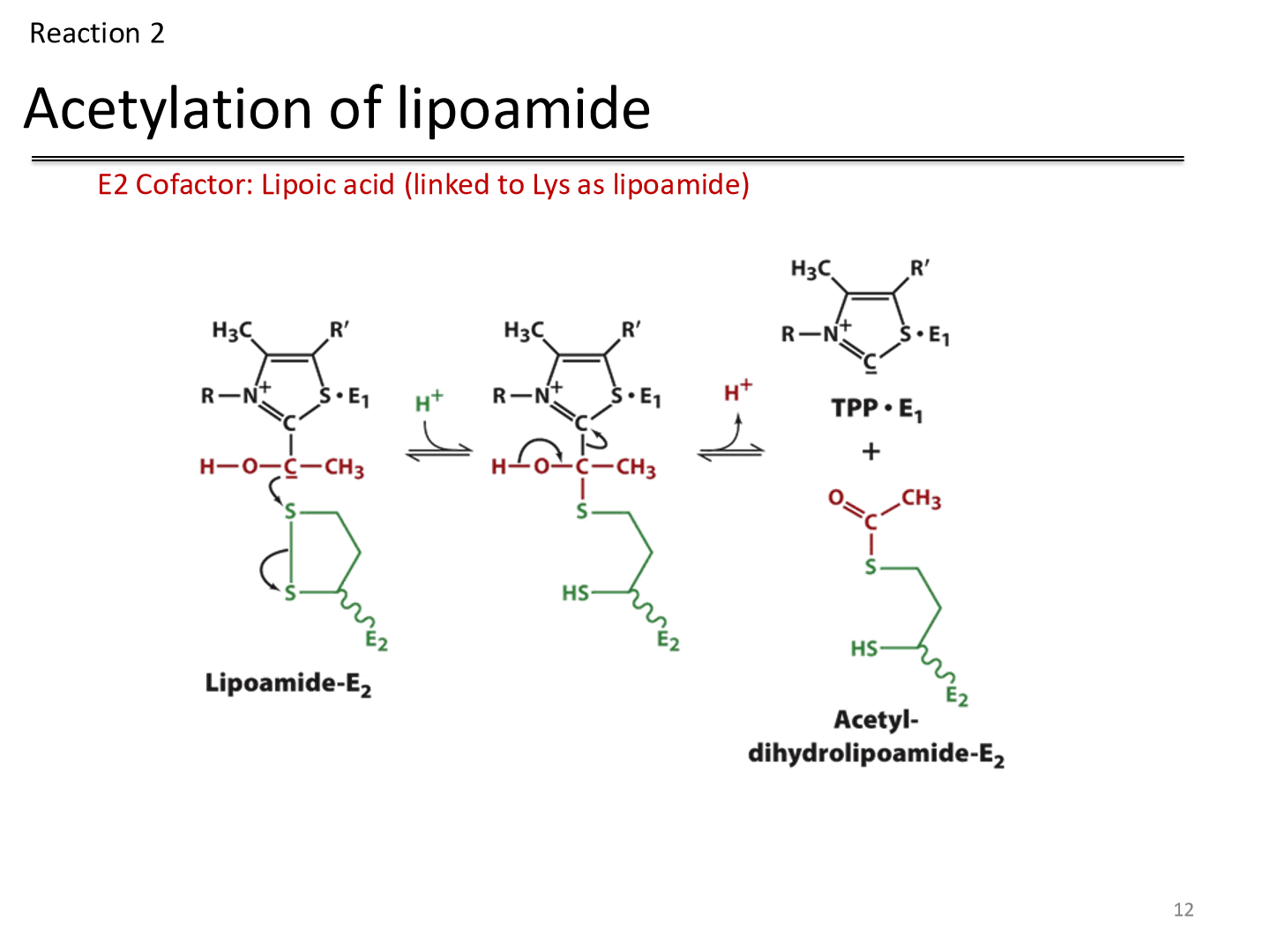
Lipoic acid
accepts the hydroxyethyl carbanion from TPP as an acetyl group after being oxidized
Reaction 3: Acetylation of CoA
Reactant: Acetyl-dihydrolipoamide
Enzyme: Dihydrolipoyl transacetylase
Cofactor: CoA —> Accepts the acetyl group from lipoamide
Product: Dihydrolipoamide
Reaction 4: Regeneration of lipoamide
Reactant: Dihydrolipoamide —> reduced by lipoamide
Enzyme: Dihydrolipoyl dehydrogenase
Cofactor: FAD
Reaction 5: Regeneration of lipoamide
Reactant: Reduced dihydrolipoamide
Cofactor: NAD+ —> reduced by FADH2
sulfhydryl groups are re-oxidized
TCA Cycle Reaction 1
Substrate: Oxaloacetate
Co-substrate: Acetyl CoA
Intermediate: Citryl-CoA
Products: Citrate + CoA—SH
Reaction 1 Enzyme
Enzyme: Citrate synthase —> catalyzes the condensation of acetyl-CoA and oxaloacetate
inhibited by citrate because citrate competes with oxaloacetate
Reaction 1 Mechanism
Rate limiting formation of acetyl-CoA enolate through binding of oxaloacetate and acetyl-CoA, stabilized by a hydrogen bond from His 274 (general Base catalysis)
Nucleophilic attack of acetyl-CoA enolate on oxaloacetate’s carbonyl carbon to produce citryl-CoA
Citryl-CoA hydrolysis
TCA Cycle Reaction 2
Substrate: Citrate
Product: Isocitrate
Reaction 2 Enzyme
Enzyme: Aconitase —> catalyzes the reversible isomerization of citrate and isocitrate, with cis-aconitate as an intermediate
iron-sulfur cluster
Reaction 2 Mechanism
dehydration in which a proton and an OH group are removed by an iron-sulfur cluster
rehydration of the double bond of cis-aconitate to form isocitrate
TCA Cycle Reaction 3
Substrate: Isocitrate
Intermediate: Oxalosuccinate (exists transiently)
Product: alpha-Ketoglutarate
produces the first CO2 and NADH of the citric acid cycle
Reaction 3: Decarboxylation of isocitrate
Enzyme: isocitrate dehydrogenase catalyzes the oxidative decarboxylation of isocitrate to alpha-ketoglutarate
catalyzes the oxidation of a secondary alcohol (isocitrate) to a ketone
inhibited by NADH
Reaction 3: Mn2+
helps polarize the newly formed carbonyl group after oxidation
Reaction 3 Mechanism
oxidation —> produces NADH from NAD+
decarboxylation —> CO2
TCA Cycle Reaction 4
Substrate: 𝛂-Ketoglutarate
Product: Succinyl CoA
produces the second CO2 and NADH of the CAC
Reaction 4: enzyme
Enzyme: 𝛂-Ketoglutarate dehydrogenase catalyzes the oxidative decarboxylation of an α-keto acid
similar to pyruvate dehydrogenase: oxidative decarboxylation to form high-energy succinyl-CoA
inhibited by NADH and succinyl-CoA
Reaction 5: Synthesis of GTP
Substrate: succinyl CoA
Product: succinate + CoA + GTP
Enzyme: Succinyl-CoA synthetase (also called succinate thiokinase) couples the cleavage of the “high-energy” succinyl-CoA to the synthesis of a “high-energy” nucleoside triphosphate
Succinyl-CoA reacts with Pi to form succinyl-phosphate and CoA.
The phosphoryl group is then transferred from succinyl-phosphate to a His residue on the enzyme, releasing succinate.
The phosphoryl group on the enzyme is transferred to GDP, forming GTP.
Reaction 6
Succinate dehydrogenase catalyzes the stereospecific dehydrogenation of succinate to fumarate and FADH2
The first reaction of the cycle which was highly exergonic, which accounts for low oxaloacetate concentrations
Reaction 7
Fumarase catalyzes the hydration of the double bond of fumarate to form malate. The hydration reaction proceeds via a carbanion transition state. OH− addition occurs before H+ addition
Reaction 8
Malate dehydrogenase catalyzes the final reaction of the citric acid cycle, the regeneration of oxaloacetate
The hydroxyl group of malate is oxidized in an NAD+-dependent reaction
Citric acid cycle regulation
the three enzymes (citrate synthase, Isocitrate dehydrogenase, Ketoglutarate dehydrogenase) regulate flux through
substrate availability
product inhibition
competitive feedback inhibition by intermediates down the cycle
There is no single flux-control point, rather flux control is distributed among several enzymes
Crucial regulators of the citric acid cycle
Acetyl-CoA
Oxaloacetate: in equilibrium with malate, and controlled by NADH/NAD+ ratio
If respiration rate increases, NADH decreases, and oxaloacetate increases and stimulates citrate synthase
NADH: isocitrate dehydrogenase and citrate synthase are inhibited by NADH