Topic 3 - Bonding: Valence Bond Theory (VBT)
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
Chemical Bond
A short range interaction between two or more species which result in the formation of a stable molecular identity.
Valence Bond Theory (VBT)
Core assumption: Electrons occupy atomic orbitals; bonds form via orbital overlap between two atoms (electron donation from each atom).
Orbital overlap as waves: Orbital overlap means that the two waves (atoms are in-phase → in-phase overlap increases amplitude → high electron density between nuclei.
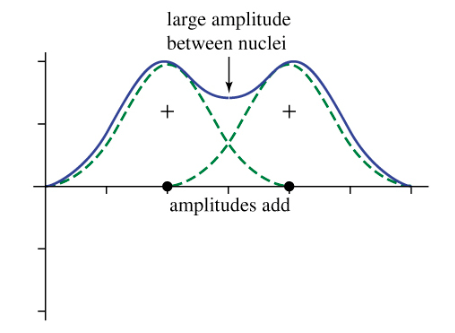
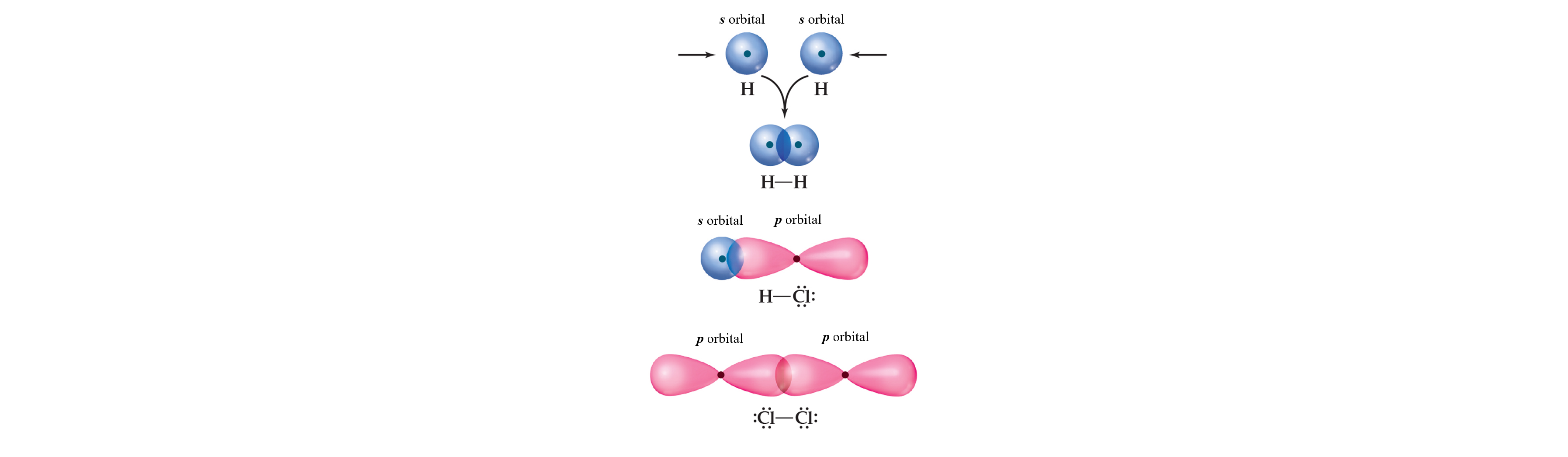
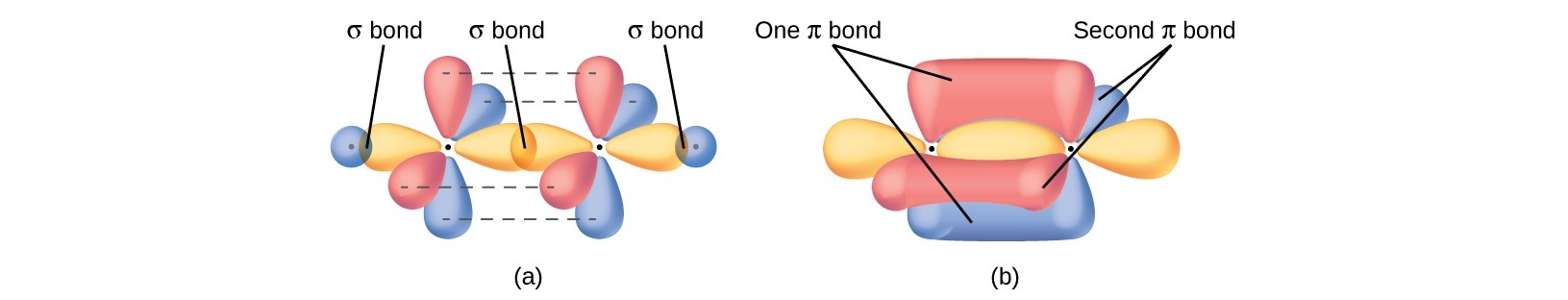
Sigma (σ) Bonds
Formation: Head-on overlap of atomic orbitals (s–s, s–p, or p–p).
Shape: Cylindrically symmetrical around the bond axis that passes through both nuclei.
Rule: Only one sigma bond can exist between any two atoms.
The second that there's like-charges that repel electrons away from each other, further orbital overlap (bonding) cannot occur in the same location.
Stronger Bond:
Type of Overlap: A head-to-head overlap maximizes the electron density.
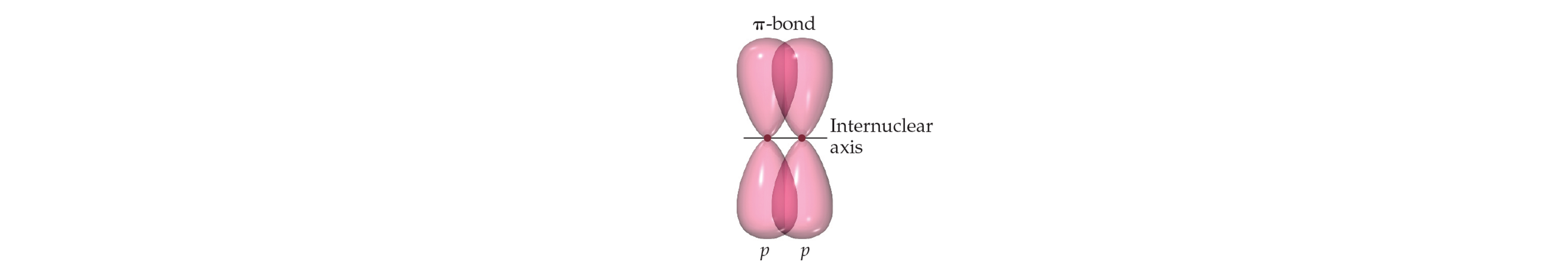
Pi (π) Bonds
Formation: Side-to-side overlap of p orbitals (above and below bond axis).
Characteristics:
Two regions of electron density (above and below nuclei).
Still considered one bond despite two regions.
Weaker Bond:
Type of Overlap:
A side-to-side overlap means less orbital overlap which minimizes the electron density.
Since electron densities are spread above and below the bond axis, there's weaker electrostatic attraction between the nuclei and bonding electrons
Orbital Alignment: A side-to-side overlap is more restricted in orientation. Any twisting/rotation would misalign the orbitals.
Single Bond
1 σ
Double Bond
1 σ + 1 π
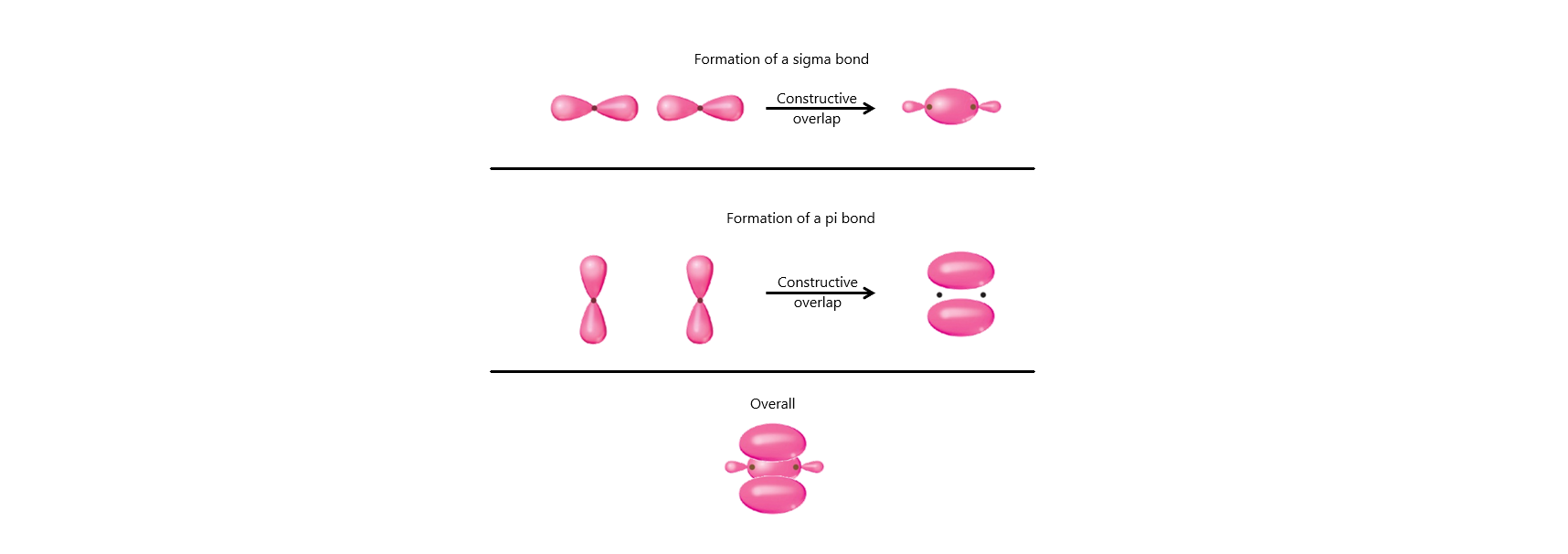
Triple bond
1 σ + 2 π
Atomic Number
Number of protons (+) in the nucleus (atomic number) determine what element it is.
The nucleus of an atom has an overall positive charge equal to the number of proton it contains.
Electrostatic Interactions
When two atoms come together to form a chemical bond, many electrostatic interactions happen simultaneously:
Nucleus (+) ↔ electrons (-) → attraction.
Nucleus (+) ↔ nucleus (+) → repulsion.
Electron (-) ↔ electron (-) → repulsion.
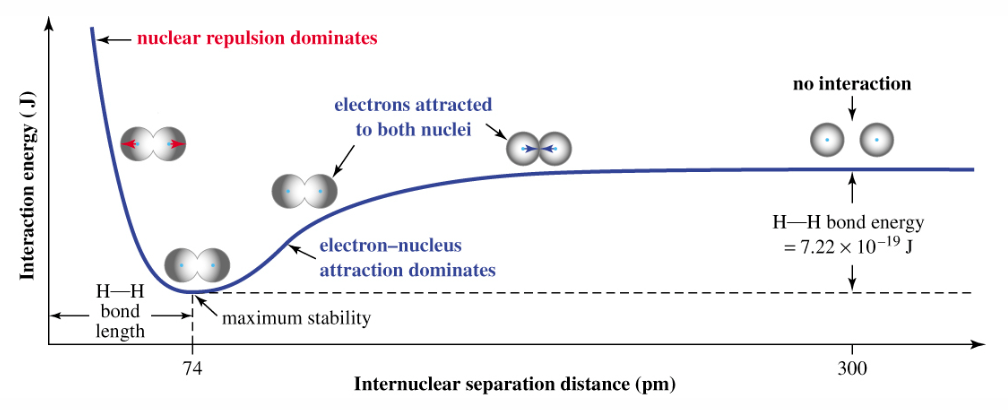
Electrostatic Interactions - Process
No interaction between atoms, the inter-nuclear distance is too large.
Atoms start coming closer together → nuclei attracted to each other's electrons → attractive forces dominate and outweighs repulsive forces → potential energy keeps dropping until a minimum.
Bonding occurs at a distance where the potential energy reaches a minimum point - point of maximum stability.
Once atoms get too close together → nucleus–nucleus repulsion dominates → energy rises sharply.
*Zero distance is impossible, nuclear repulsion is infinitely strong!
Total Potential Energy
Energy of the interaction of two atoms; all attractive forces + all repulsive forces
Bond Length
Internuclear distance at minimum energy - where bonding occurs.
Bond Energy
Energy needed to break a chemical bond, that is the energy difference between non-interacting atoms and atoms at minimum energy; always positive.
Bond Order
Definition: Number of chemical bonds between a pair of atoms.
Relationships: Higher bond order → shorter bond length → higher bond energy → greater stability.
Trends: Smaller atoms (e.g., H₂) → shorter bonds, as the valence electrons are in orbitals that are physically closer to the nucleus.