Biological molecules
0.0(0)
Card Sorting
1/101
There's no tags or description
Looks like no tags are added yet.
Last updated 1:00 PM on 4/6/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
102 Terms
1
New cards
2
New cards
What chemical elements make up a lipid and carbohydrate
C H O
3
New cards
What chemical elements make up a protein
C H O N S
4
New cards
What chemical proteins make up nucleic acids
C H O N
5
New cards
Define monosaccharide
A single sugar molecule
6
New cards
Draw alpha Glucose
C6hH2O6
7
New cards
Draw Beta glucose
C6h1O6
8
New cards
Difference between Beta and Alpha glucose?
In Alpha glucose the OH on carbon 1 is at the bottom and in Beta glucose the OH is at the top.
9
New cards
what do you get when you bond 2 alpha glucoses together
a disaccharide
10
New cards
What is the bond from a disaccharide called
1-4 glycosidic bond
11
New cards
How is the alpha 1-4 glycosidic bond formed
By a condensation reacation
12
New cards
Draw ribose
C5H10)5
13
New cards
Difference between ribose and glucose
Ribose is a pentose sugar and glucose is a hexose sugar.
14
New cards
Alpha glucose + Alpha glucose =
Maltose
15
New cards
Beta glucose + galactose =
Lactose
16
New cards
Alpha glucose + Fructose =
Sucrose
17
New cards
Draw a diagram of water and include the correct labels
(must include the hydrogen bond lable and water is polar label)
18
New cards
Why is water polar?
Oxygen and hydrogen do not share electrons equally when joined by a covalent bond. So you get an uneven distribution of charge around the molecule.
19
New cards
Property of water (BP)
Importance for living organism
Example in nature
Importance for living organism
Example in nature
Unusually high boiling point
A lot of energy needed to break hydrogen bonds
Stable water temperature for aquatic animals; less energy spent on temperature control
A lot of energy needed to break hydrogen bonds
Stable water temperature for aquatic animals; less energy spent on temperature control
20
New cards
Property of water (Ice
Ice is less dense than water
Ice forms insulating layer above water below
Ice floats on the surface of the sea and is a habitat.
\
Ice forms insulating layer above water below
Ice floats on the surface of the sea and is a habitat.
\
21
New cards
Property of water (Cohsv Prop)
Importance for living organism
Example in nature
Importance for living organism
Example in nature
Cohesive properties
Creates a high surface tension for insects.
Easier for plants to draw up\[ water for the roots.
Creates a high surface tension for insects.
Easier for plants to draw up\[ water for the roots.
22
New cards
Property of water (adh prop)
Importance for living organism
Example in nature
Importance for living organism
Example in nature
Adhesive properties
Moves up the xylem into the plant
Water is attracted to other molecules
Moves up the xylem into the plant
Water is attracted to other molecules
23
New cards
Property of water (solv for pol molc)
Importance for living organism
Example in nature
Importance for living organism
Example in nature
Acts as a solvent for polar molecules
Allows mineral ions to be transported
Glucose in the blood.
Allows mineral ions to be transported
Glucose in the blood.
24
New cards
Property of water (transport med)
Importance for living organism
Example in nature
Importance for living organism
Example in nature
Transport medium
Allows the transport of soluble substances
Transporting substances around the blood.
Allows the transport of soluble substances
Transporting substances around the blood.
25
New cards
property of water
Importance for living organism
Example in nature
Importance for living organism
Example in nature
Coolant
Evaporating for cooling
Sweating in animals
Evaporating for cooling
Sweating in animals
26
New cards
Property of water (stable temp)
Importance for living organism
Example in nature
Importance for living organism
Example in nature
Temperature remains relatively stable
Creates a stable temperature for enzymes
Metabolic reactions
\
Creates a stable temperature for enzymes
Metabolic reactions
\
27
New cards
Property of water (Cap ac)
Importance for living organism
Example in nature
Importance for living organism
Example in nature
Capillary action
allows water to move up narrow vessels
e.g xylem
allows water to move up narrow vessels
e.g xylem
28
New cards
what is a Polysaccharide
Polymers that are formed by many monosaccharides joined by glycosidic bonds formed by condensation reactions.
29
New cards
What is amylose made of
Alpha 1-4 glycosidic bonds
30
New cards
Structure of amylose
coiled structure which is compact
31
New cards
Advantage of the structure of amylose
Perfect for carbohydrate storage
Doesn’t affect osmosis
insoluble (property)
Doesn’t affect osmosis
insoluble (property)
32
New cards
What is amylopectin made of
Alpha 1-6 glycosidic bonds
33
New cards
Structure of amylopectin
Branched structure
34
New cards
advantage of the structure of amylopectin
easy to hydrolyse
35
New cards
What hydrolyses amylopectin and why
enzymes hydrolyse amylopectin and turn it into glucose so it can be used for aerobic respiration to make ATP
36
New cards
Structure of glycogen and why is it structured like that?
Contains many branches and is compact due to the alpha 1-4 and alpha 1-6 glycosidic bonds.
37
New cards
Properties of glycogen
Insoluble
doesn’t affect water potential
compact
doesn’t affect water potential
compact
38
New cards
where is glycogen stored
In the liver
39
New cards
What is cellulose made from
Beta glucose
40
New cards
What is special about the structure of cellulose
Every second beta glucose orientates 180°
41
New cards
Structure of cellulose
Beta glucose chains form cross links between each other.
42
New cards
What are the cross links of cellulose made up of
Hydrogen bonds.
43
New cards
What do crosslinks form and what does that structure form and so on
crosslinks form microfibrils microfibrils form macrofibrils and macrofibrils from cellulose fibres
44
New cards
Properties of cellulose
High tensile strength
Insoluble
Doesn’t affect water potential
Inert
Insoluble
Doesn’t affect water potential
Inert
45
New cards
What is glucose stored as
Starch
46
New cards
What is starch made of
Amylose and amylopectin
47
New cards
What does a cell do when it needs glucosse
It hydrolysis
48
New cards
what is a triglyceride made of (draw it)
Glycerol and 3 fatty acids
49
New cards
what are the bonds between the fatty acids and glycerol called
Ester bonds
50
New cards
What makes a fatty acid unsaturated
A double bond
51
New cards
Draw a saturated and unsaturated fatty acid
\~Not bent and bent
52
New cards
properties of unsaturated fatty acids
less dense so they are liquid as there is more space between them
53
New cards
What is a phospholipid made of
What bond does it contain
What bond does it contain
a glycerol two fatty acids and a phosphate group
Ester bond
Ester bond
54
New cards
Properties of a phosphollipid
The heads are hydrophilic and the tails are hydrophobic
55
New cards
What is found in between the tails of a phospholipid
Cholesterol
56
New cards
Role of lipids (Mem form & ______n of HP barriers)
Explanation and examples
Explanation and examples
Membrane formation and creation of hydrophobic barriers
Phospholipids form the cell membrane
Phospholipids form the cell membrane
57
New cards
Role of lipids (HRMN PRD)
Explanation and examples
Explanation and examples
Hormone production
Produces testosterone
Produces testosterone
58
New cards
Role of lipids (ELCTRC INSLTN)
Explanation and examples
Explanation and examples
Electrical Insulation
Necessary for impulse transmission ( e.g Myelin sheath)
Necessary for impulse transmission ( e.g Myelin sheath)
59
New cards
Role of lipids (WTR PRFNG)
Explanation and examples
Explanation and examples
Water proofing
Birds feathers and plant leaves.
Birds feathers and plant leaves.
60
New cards
Role of lipids (CSHNG PRTCTN)
Explanation and examples
Explanation and examples
Cushioning/protection
Protects vital organs e.g the heart and kidneys.
Protects vital organs e.g the heart and kidneys.
61
New cards
Role of lipids b(BNCY)
Explanation and examples
Explanation and examples
buoyancy
Aquatic animals e.g wales
Aquatic animals e.g wales
62
New cards
Role of lipids (ENGY STR)
Explanation and examples
Explanation and examples
Energy store
Respiratory systems
Respiratory systems
63
New cards
Role of lipids (ADNG ABSRBTN)
Explanation and examples
Explanation and examples
Aiding absorbption
soluble molecules e.g vitamin D&A
soluble molecules e.g vitamin D&A
64
New cards
Role of cholesterol
Regulates fluidity
65
New cards
What are proteins made of
Amino acids
66
New cards
Draw and label an amino acid and then draw two repeating units a and label
CHON
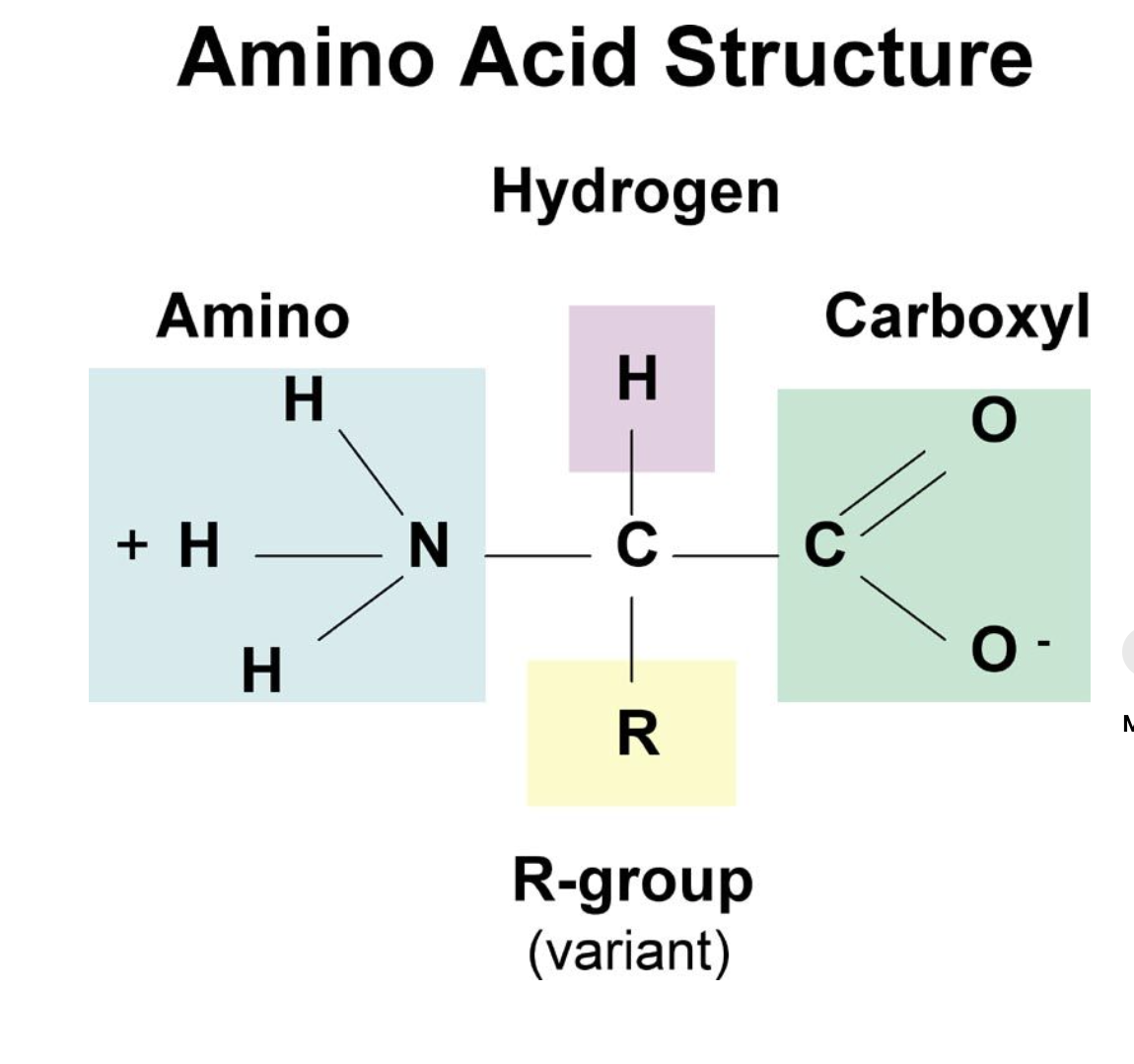
67
New cards
What is the bond between 2 amino acids called
Peptide bond
68
New cards
Primary structure
The sequence of amino acids bonded by peptide bonds
69
New cards
Secondary structure
Folding of the polypeptide chain; held in place with hydrogen bonds; alpha helix or beta pleated sheets
70
New cards
Tertiary structure
Further folding of the polypeptide chain
held in place by hydrogen bonds; disulfide bridges (s=s) ionic bonds; amino acids with hydrophobic groups orientate towards the protein and amino acids with hydrophilic R groups orientate away from the protein.
held in place by hydrogen bonds; disulfide bridges (s=s) ionic bonds; amino acids with hydrophobic groups orientate towards the protein and amino acids with hydrophilic R groups orientate away from the protein.
71
New cards
Quaternary structure
More than 1 polypeptide chain
72
New cards
What do Globular proteins do
Provide a metabolic role
73
New cards
What type of proteins are globular
Enzymes or hormones
74
New cards
Properties of globular proteins
compact
Spherical (usually)
soluble
folding keeps hydrophobic R groups away from environment
Spherical (usually)
soluble
folding keeps hydrophobic R groups away from environment
75
New cards
What is a conjugated protein
A globular protein with a prosthetic group
76
New cards
what is a prosthetic group
a non protein group
77
New cards
Why is insulin soluble
So it can be transported around the blood
78
New cards
How can insulin be detected by its receptor
its very specific shape
79
New cards
Structure of haemoglobin
2 alpha polypeptide chains and 2 beta polypeptide chains
4 haem groups made of iron which make it easier to bind to oxygen.
4 haem groups made of iron which make it easier to bind to oxygen.
80
New cards
Why does Haemoglobin change shape
To accommodate more oxygen
81
New cards
What does catalase do
Catalyses active site and breaks down haem groups
82
New cards
Where are fibrous proteins found
Collagen in ears , keratin in nails and hair elastin in blood vessels.
83
New cards
What structure does a fibrous protein not have
tertiary structure
84
New cards
What makes a fibrous protein insoluble
High proportion of amino acids with hydrophobic R groups
85
New cards
What do fibrous proteins form
Long chains which run parallel to each other.
Linked by cross - bridges forming strong structures.
Linked by cross - bridges forming strong structures.
86
New cards
Keratin
wheres it found
property
what does it contain
wheres it found
property
what does it contain
Flexible in hair and nails
Hard and tough
contains di - sulfide bridges
Hard and tough
contains di - sulfide bridges
87
New cards
Elastin
Wheres it found
Property
Wheres it found
Property
Walls of blood vessels
Stretches and recoils back to its original shape.
Stretches and recoils back to its original shape.
88
New cards
collagen
wheres it found
whats it made of
property
wheres it found
whats it made of
property
Found in skin, ligaments and nervous system
3 polypeptide chains entwined and wrapped and held by H+ bonds
flexible but doesn’t stretch.
3 polypeptide chains entwined and wrapped and held by H+ bonds
flexible but doesn’t stretch.
89
New cards
symbol of calcium Ion
Ca2+
90
New cards
Symbol of sodium Ion
Na+
91
New cards
symbol of potassium ion
K+
92
New cards
Symbol of hydrogen Ion
H+
93
New cards
Symbol of Ammonium Ion
NH4+
94
New cards
Nitrate ion symbol
\-No
95
New cards
HYdrogen carbonate symbol
HCO3-
96
New cards
Phosphate ion symbol
PO^34-
97
New cards
Hydroxide ion symbol
OH-
98
New cards
Chloride ion symbol
Cl-
99
New cards
How to calculate RF value
distance travelled by component/distance travelled by solvent
100
New cards
What do you have to do to a calibre
Calibrate to 0 using a blank