chapter 3- alkenes
1/42
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
43 Terms
what are alkenes?
unsaturated hydrocarbons
general formula is CnH2n
contain at least 1carbon-carbon double bond
what is the shape around the carbon atom of the double bond?
trigonal planar
because there are three regions of electron density around each of the carbon atoms
the three regions repel each other as far apart as possible, so the bond angle around each carbon atom is 120
all of the atoms are in the same plane
what bonds does the c=c double covalent bond consist of?
one sigma bond
one pi bond
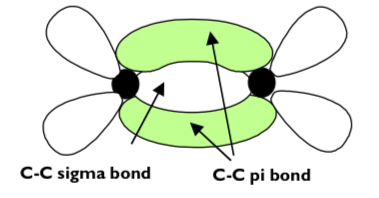
how is the pi bond formed?
by a sideways overlap of 2 p-orbitals on each carbon atom
this forms a pi bond above and below the plan of the molecule
the pi bond is weaker than the sigma bond
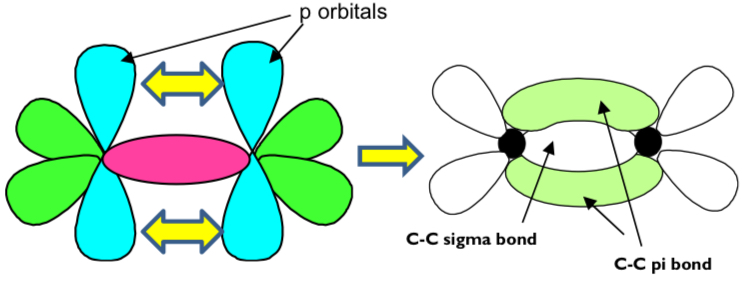
how is the sigma bond formed?
overlap of orbitals directly between the bonding atoms
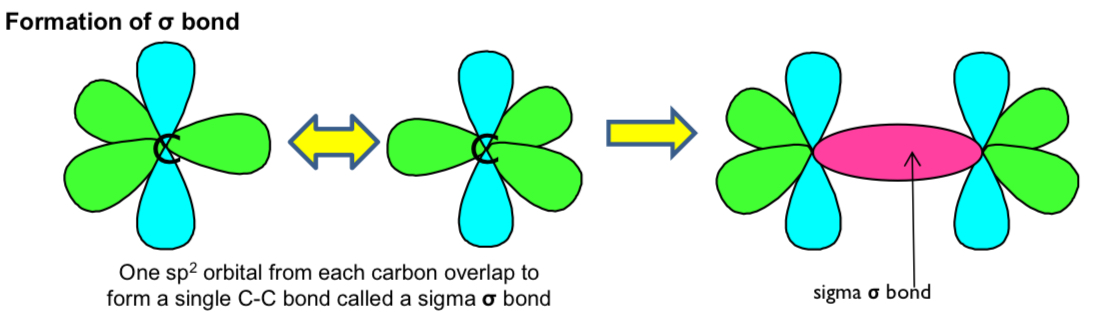
rotation on sigma and pi bonds?
there can be a rotation around a sigma bond\
there is a restriction rotation about a pi bond
characteristics of the pi bond?
they are exposed and therefore have high electron density
why are pi bonds more vulnerable to attack?
because of their exposure and high electron density
what attacks the pi bonds does?
species that ‘like’ electrons called electrophiles
electrophile meaning?
positive ions, or molecules with a partial positive charge, that are attracted to the c=c bond (region of electron density)
electron pair acceptor
why are alkenes reactive?
the c=c bond has a greater electron density and therefore a greater negative charge
therefore, molecules can react with alkenes by accepting a pair of electrons from the c=c bond
electrophiles are attracted to the c=c bond
most reactions with alkenes involve electrophiles
testing for alkenes?
add bromine water to the alkene at room temperature
observation: orange colour of bromine water will decolourise to colourless
it forms a dibromoalkane
what is more reactive: alkanes or alkenes?
alkenes because of the double bond
why is there stereoisomerism in alkenes?
because of the presence of double bonds
unlike single bonds, double bonds are rigid and you cannot rotate around them
their single bonds allow free rotation
so alkenes display stereoisomerism at the double bonds
also, each carbon atom of the double bond is attached to 2 different groups
definition of stereoisomers?
same structural formula
different arrangement of atoms in space/different spatial arrangement of atoms
definition of E/Z isomerism?
example of stereoisomerism, in terms of restricted rotation about a double bond and the requirement for 2 different groups to be attached to each carbon atom of the carbon=carbon double bond
definition of cis-trans isomerism?
special case of E/Z isomerism in which 2 of the substituent groups attached to each carbon atom of the c=c group are the same
what is the reaction of an alkene with bromine/chlorine an example of?
electrophilic addition
what happens in the reaction between ethene (an alkene) and bromine? step 1
1) as the Br2 molecule approaches the alkene, the pi bond electrons repel the electron pair in the Br-Br bond, this then induces a dipole
the pi bond repels the electron pair, because the pi bond is a region of high electron density- electrons are negative in the alkene, and in the br bond, like charges repel
this induces a dipole, so the Br2 becomes polar and electrophilic
what happens in the reaction between ethene and bromine step 2?
the bromine delta + is attracted to the electron rich pi bond
the 2 electrons in the pi bond come out to make a covalent bond with the positive bromine of the br2
the br-br bond breaks heterolytically
one Br attaches to one of the carbon atoms, and a bromide ion is formed with the other
what happens in the reaction between ethene and bromine, step 3?
the bromide ion contains a lone pair
it acts as a nucleophile and is attracted to the positive carbon on the carbocation, forming a new covalent bond
overall, there is an addition of br2 across the alkene
why does the pi bond give out electrons?
because the pi bond has a high electron density and is unstable
they are ‘looking’ for a way to get more stable
why does the br-br bond break heterolytically?
because one of the bromines is now covalently bonded to the carbon
electrophilic addition of water- characteristics?
only the first stage is an electrophilic addition reaction
needs a catalyst (phosphoric acid absorbed onto silica)- used to make ethanol
high temperature of 400C and Pressure 60atm
concentrated sulfuric acid is used in the lab, hydration reaction
what would happen to the addition of hydrogen bromide if the alkene is unsymmetrical (markownikoff’s rule)?
it can lead to 2 isomeric products because there are 2 different possibilities where the bromine could attach to the alkene- either side of the double bond
do the 2 products form in equal amounts?
no
one is called major and one is called minor
what rule do you use to determine whether it is a major or minor product?
markownikoff’s rule
in most cases, the bromine will be added to the carbon with the fewest hydrogens attached to it
example of markownikoff’s rule with the formation of bromobutane from but-1-ene and hydrogen bromide?
the 2-bromobutane forms much more often (90%) and is called the major product
the other possibility- 1-bromobutane- is called the minor product and forms much less (10%)
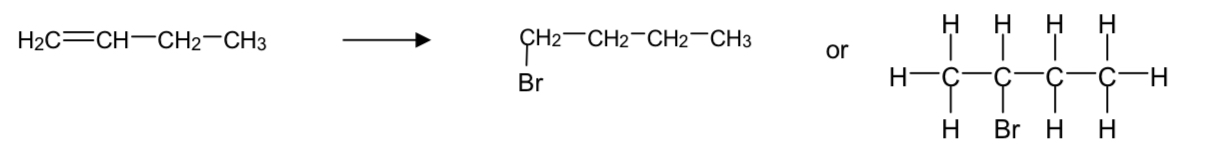
what catalyst is used in the electrophilic addition of an alkene and hydrogen?
(to form an alkane)
nickel catalyst
also known as a hydrogenation reaction
what catalyst is used in the electrophilic addition of water?
(to form an alcohol)
an acid catalyst, such as phosphoric acid or sulfuric acid catalyst
what happens in addition polymerisation?
the alkene molecules add to themselves to form long chain molecules
the double bond in the alkene opens to bond to the next monomer molecule
what is the original alkene molecule called in addition polymerisation?
monomer
how do chains form?
when the same basic unit is repeated over and over
what is the polymer of ethene called?
(poly)ethene
why are polyalkenes unreactive?
because of the strong c-c and c-h bonds
what are the bonds in polymer chains?
strong covalent bonds
between different polymer chains there are weaker intermolecular forces (london forces and permanent dipole forces)
what does increased chain length lead to in polymers?
increasing density, melting point and hardness
uses of polymers?
they are unreactive which makes them useful for manufacturing everyday plastic products
however, due to them being unreactive, it means they are not biodegradable
therefore, they may go to landfill
how to dispose polymers?
waste polymers could be used as organic feedstock to produce plastics and other organic compounds
recycled
some are combusted to produce energy for other industrial processes
what happens during combustion of polymers?
they can release toxic gases which must be removed to reduce the impact of the environment
advantage of combustion?
advantage: energy used to produce electricity
disadvantages of combustion?
formation of hcl
releases co2/gases that cause global warming
benefits to the environment of development of biodegradable and photodegradable polymers?
reduced long-term pollution
alleviating problems from disposal of persistent plastic waste
less harm to wildlife
reduced landfill use