Levels of Protein Structure & Their Characteristics
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
30 Terms
Why is the shape of a protein important?
Provides function to a protein
Proteins are EVERYWHERE in the human body, ranging from transport proteins to enzymes - no shape is one-size-fits-all!
Provides specific solubility to a protein
Denaturation
Protein losing its shape, and therefore entire function/activity and solubility capabilities
Disruption of weak interactions that would normally stabilize the structure
Causes denatured proteins to clump together, causing more harm than simply losing their function
Examples of diseases involving denatured/misfolded proteins
Sickle cell anemia
Huntington’s disease
Parkinson’s disease
Alzheimer’s disease
Osteogenesis Imperfecta
Prion diseases: Mad cow disease, Creutzfeldt-Jakob disease, Kuru disease
Primary Structure
Unique amino acid sequence linked by peptide bonds, forming a polypeptide
Unfolded but has blueprints for how it will fold in the future
Peptide Bond Characteristics
Partial double-bond character between terminal ends of each amino acid (NH+ group and COO- group)
Written N- to C- terminal (L-R)
UNCHARGED
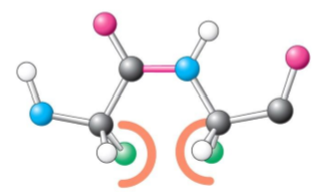
Cis side chain
Relative to a peptide bond between two amino acids, the R side chain is on the same side on one amino acid as the other amino acid’s R side chain
More likely to have steric hindrance (slowing of chemical rxn)
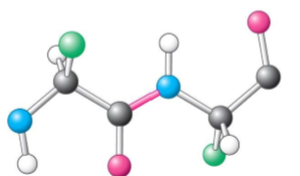
Trans side chain
Relative to a peptide bond between two amino acids, the R side chain is on the same side on one amino acid as the other amino acid’s R side chain
Less likely to have steric hindrance (slowing of chemical rxn)
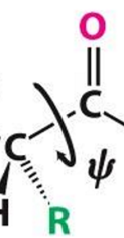
Torsion angle
Measure of rotation for phi and psi bonds between peptide bonds
Preferred angle is depicted by a Ramachandran plot
Most favored angles = dark green regions
Least favored angles = light green regions
Most conformations are unfavorable due to steric exclusions!
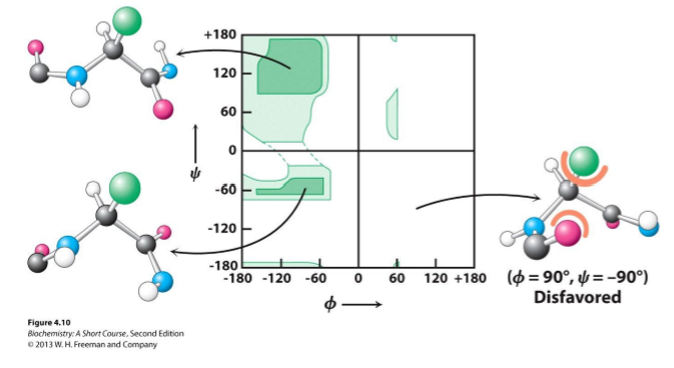
Steric exclusion
Phenomenon where a molecule is too large to fit next to another molecule
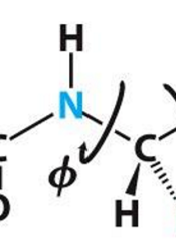
Phi bonds Φ
N-C(alpha) bonds
Psi bonds Ψ
C(alpha)-C bond
Secondary Structure
Localized, small 3D structure
Stabilized by H bonding of peptide backbone
Alpha helixes
Beta pleated sheets
Loops
Turns
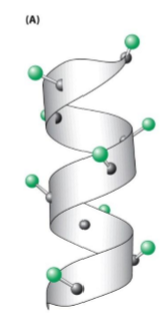
Alpha helix
Stabilized by H bonding
3.6 amino acids per turn
Unlikely to have steric exclusions (V, T, I)
Unlikely to have H bond donors (S, D, N)
Unlikely to have phi rotation for P due to lack of NH group
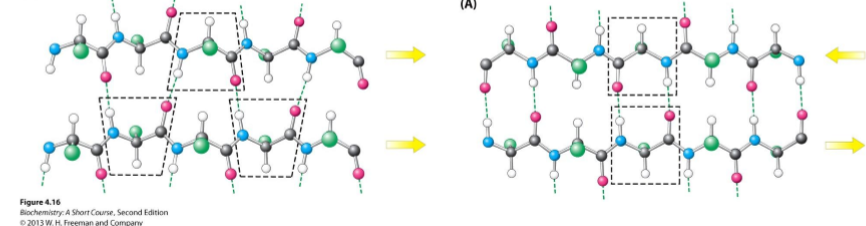
Beta pleated sheet
2 or more adjacent polypeptide strands called “B-strands”
Stabilized by H bonding between C=O and N-H groups
Can be parallel or antiparallel
Adopts a twisted shape, but is still beta pleated sheets
Loops and Turns
Changing of direction of polypeptides, usually on the edge or surface of a protein
Stabilized by H bonding
Tertiary Structure
Single completely folded 3D protein structure - may end structure here if fully functional
Stabilized by interactions between R groups and interaction of amino acids far apart in primary structure
Disulfide bonds are created
Functional units:
Motifs
Domains
Disulfide bonds
Formed by the OXIDATION of 2 CYSTEINE amino acids, causing covalent cross-linking of polypeptides
VERY strong
Stabilizes tertiary AND quaternary structure
Motifs
Special characteristics of the structure of the protein itself
Combination of multiple secondary structures
Domains
Sections of proteins with a specific function
A single polypeptide may have 1+ domains depending on its final function
Common domains:
RNA/DNA binding
Catalytic activity
Transcriptional activation
Protein-protein interaction
Cytoskeleton interaction
Typically represented in colored BOXES
Quaternary Structure
More than 1 polypeptide interacting
Each polypeptide chain is called a SUBUNIT
Stabilized by interaction of side chains
NOT ALL PROTEINS HAVE THIS LEVEL OF ORGANIZATION
Subunit
Single polypeptide in a protein with quaternary structure
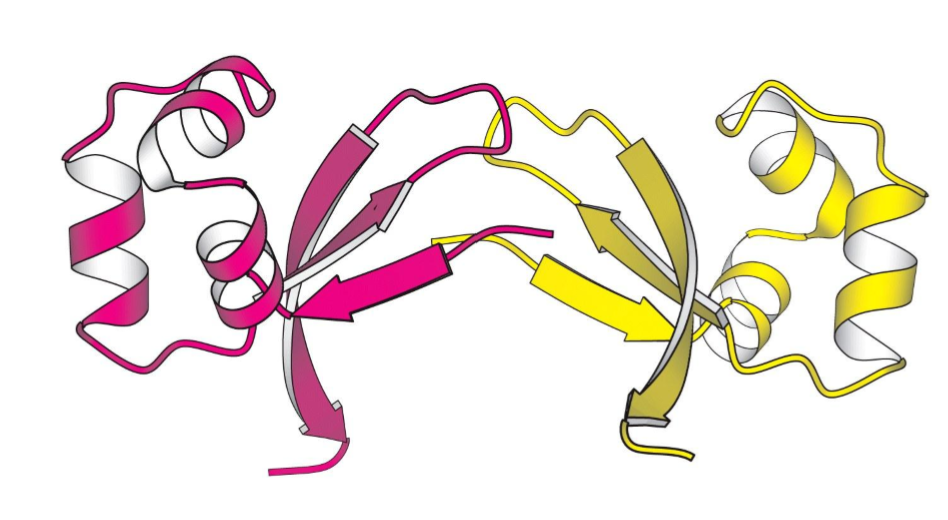
Dimer
2 subunits
Homodimer
Heterodimer
Homodimer
2 identical peptide subunits
Heterodimer
2 different peptide subunits
May be encoded by different genes, but nonetheless comes together to make 1 protein subunit
“Complex” quaternary structure
MANY subunits stabilized together to make 1 LARGE protein
3 CLASSES OF PROTEINS
Fibrous proteins
Globular proteins
Membrane proteins
Fibrous proteins
Relatively simple and LINEAR in shape
Functions to provide structural support
Keratin
Collagen
Cytoskeleton proteins (intermediate filaments)
Muscle proteins (myosin & tropomyosin)
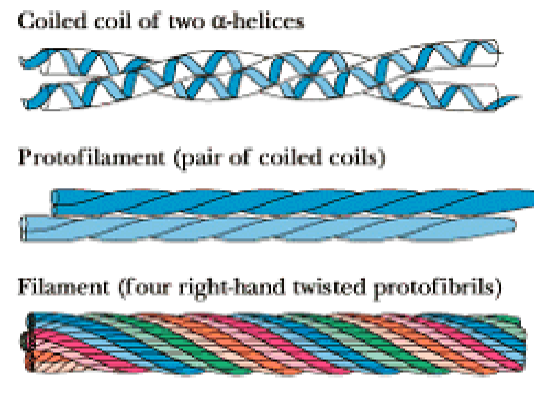
Collagen
Fibrous protein
Component of skin, bone, tendons, cartilage, teeth, etc.
3 intertwined helical polypeptide chains, forming a superhelical cable
GLY - GLY - PRO sequence is commonly repeated throughout its structure
Stabilized by steric repulsion of the PRO rings
Chains interact by H bonding
Defects in collagen structure:
Osteogenesis imperfecta
Scurvy
Globular proteins
Compact and roughly spherical in shape
Water SOLUBLE
Functions to provide enzymatic activity and signaling capabilities
Myoglobin
Hemoglobin
Membrane proteins
Proteins associated with cellular membranes
Channels for chemical transport across the membrane
Carrier proteins for chemical transport across the membrane
Have to interact with hydrophobic AND hydrophilic membrane sections
Water INSOLUBLE
Exterior of protein = mostly hydrophobic amino acid chains
Interior of protein = mostly hydrophilic amino acid chains
Like the phospholipid membrane!