Steam distillation and solvent extraction
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
34 Terms
Steam distillation
What is the term used for this particular method of extracting essential oils?
The safety tube is placed well below the level of water in the steam generator.
The safety tube will release pressure/ steam.
Explain in detail one safety feature of the apparatus used for the steam distillation of clove oil?
so the cloves can be extracted at a temperature below its boiling point
Why is clove oil extracted using steam distillation?
Whole cloves contain more clove oil than ground cloves.
Why is it preferable to use whole cloves instead of ground cloves in the pear-shaped flask?
The product has a pale milky appearance.
It is called an emulsion.
Describe the product’s appearance in this experiment and give the scientific name for it.
Solvent extraction
Name the process used to isolate and collect a pure sample of the clove oil
cyclohexane
Name a suitable organic solvent for use in the liquid-liquid extraction of clove oil.
The emulsion is shaken up in a separating funnel with the non-polar solvent cyclohexane.
A drying agent (like magnesium sulfate) is added.
The clove oil is evaporated off in the fume cupboard.
Explain how clove oil is separated from water in solvent extraction.
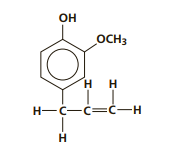
An aromatic compound containing a benzene ring.
What is eugenol? Draw the structure of eugenol
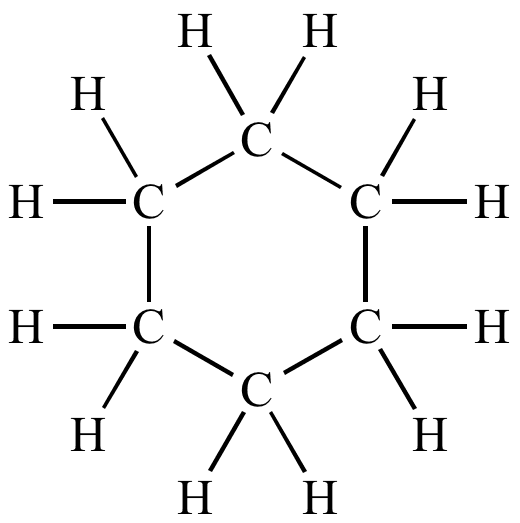
Draw the structure of cyclohexane
Open the tap (stopcock) of separating funnel to release the pressure of any vaporised liquid.
Name a safety process to be carried out during the solvent extraction of clove oil
An emulsion is a mixture of oil droplets which are suspended in water.
What is an emulsion?
MUST UNDERSTAND SOLUTION
ANSWER IS 3.15%-3.2%
PRACTICE QUESTION:
“Steam distillation and liquid-liquid extraction were used to isolate 0.15cm3 of clove oil (density 1.05g/cm3) from 5.0g of cloves. What was the percentage yield, by mass, of clove oil?”
Water
What substance distilled across along with the clove oil?
Immiscible liquids are substances that DO NOT dissolve in each other.
Explain the term immiscible liquid
Clove oil (eugenol).
Comes from the clove plant.
What substance did you isolate by steam distillation? What plant did it come from?
A separating funnel has a stopper whereas a dropping funnel has no stopper.
What is the difference between a separating funnel and a dropping funnel?
Cyclohexane is less dense than water.
Why does the cyclohexane (organic layer) float on top of the emulsion (aqueous layer)?
until the cyclohexane (organic layer) and the emulsion (aqueous layer) have reseparated.
In solvent extraction, how long do we let the separating funnel stand for? (after shaking)
Allow the bottom (aqueous layer) to flow into a beaker marked aqueous layer. Then allow the top layer (organic layer) to flow into a conical flask.
How do we separate the organic layer (cyclohexane) and the aqueous layer (emulsion)
Repeat the washing multiple times.
How do you extract the maximum amount of oil from the emulsion in solvent extraction?
by adding the drying agent magnesium sulfate. (add as much as needed- it will be filtered off)
In solvent extraction after all the washing has been complete, how do we remove the excess water?
Pour it through filter paper into your weighed out conical flask.
In solvent extraction, how do we then get rid of the drying agent magnesium sulfate?
Pour in 10cm3 of cyclohexane into beaker. Then filter through fluted filter paper.
How do you collect the residual oil left in the beaker of magnesium sulfate?
The surface area of the filter paper is greater then what it would be if the filter paper was folded in the usual way.
Why is fluted filter paper so effective for filtering substances?
Place the conical flask in a water bath.
Place water bath on hot plate and evaporate off the volatile cyclohexane solvent.
Allow the conical flask to stand overnight in the fume cupboard to allow any remaining cyclohexane to evaporate.
How do you evaporate off the volatile cyclohexane solvent?
This could boil off the clove oil and the cyclohexane solvent.
In solvent extraction, why do we not evaporate off the cyclohexane by placing the conical flask directly on the hot plate?
Cyclohexane is a flammable solvent.
Why would you never heat cyclohexane using a bunsen burner?
It has a burning effect on your skin.
Why do you not allow the eugenol (clove oil) to come into contact with your skin?
The steam trap collects any steam that has condensed to water. If not present, the flask containing the cloves would quickly fill up with water.
Explain the function of the steam trap.
Disconnect the steam generator first to prevent suckback. THEN turn off heat.
What do you do to prevent suckback when finishing the steam distillation experiment?
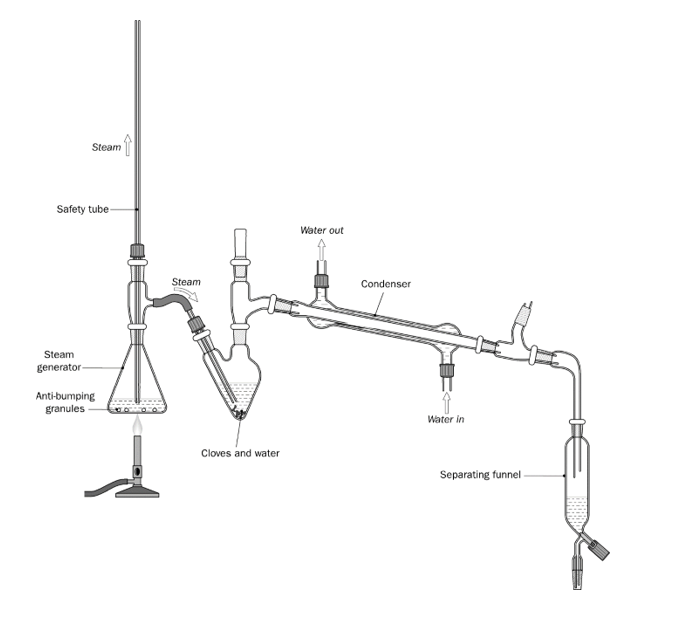
Draw the apparatus for the steam distillation of clove oil.
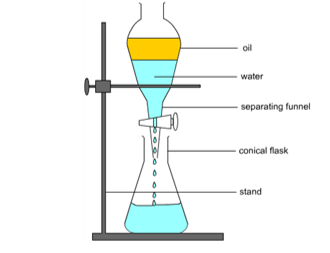
Draw the apparatus for the solvent extraction
Anti-bumping granules
What do you add to the water in the steam generator?