Leaving Groups, Steric Effects, Substrate Restrictions, Nucleophilicity.
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
What is the leaving group ability of Alkyl Halides?
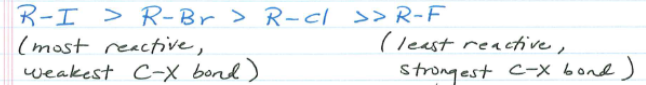
What must a leaving group be?
A stable anion. More stable, more effective.
What don’t act as leaving groups in SN2 reactions?
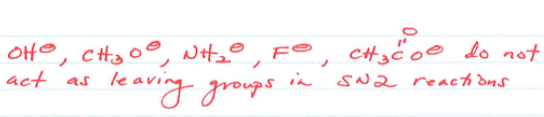
What are steric effects in these reactions?
SN1 and E1: Favor bulky, sterically hindered substrates due to carbocation stability.
SN2 and E2: Favor less hindered substrates, as steric bulk can impede nucleophilic attack or proton abstraction.
Substrate being attacked must be accessible.
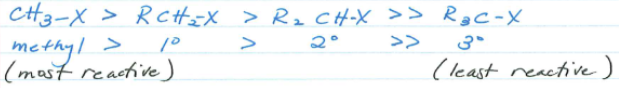
Substrate Restrictions of Sn2 Reactions.
Tertiary, Vinylic and Aryl Halides.
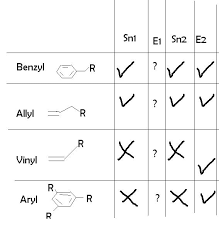
What is nucleophilicity?
A measure of the nucleophilic strength or reactivity of a nucleophile (base)

Nucleophilic strength of Neutral Base vs corresponding Conjugate Base.
Neutral bases are weaker.
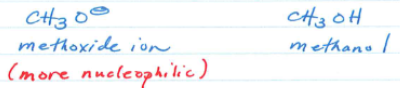

When comparing nucleophilic atoms in the same row, what are better nucleophiles?
Stronger Bases.

List very good, good, fair, and weak nucleophilic atoms.
