1 shapes of molecules, electronegativity and polarity
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
electron pair repulsion theory
the shape of a molecule is determined by the e- pairs surrounding the central atom.
e- pair repel as far as possible
lone pairs repel more than bonding pair (closer to nucleus)
what is the shape of a molecule with 2 bonding pairs and 0 lone pairs?
linear with a bond angle of 180° (e.g. CO2).
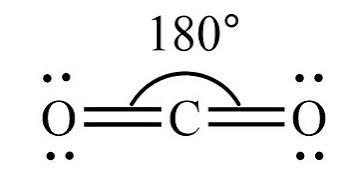
what is the shape of a molecule with 3 bonding pairs and 0 lone pairs?
trigonal planar with a bond angle of 120° (e.g. BF3).
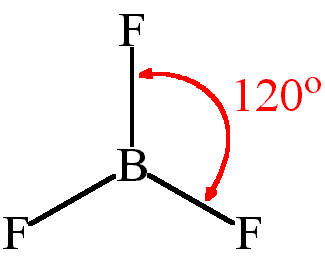
what is the shape of a molecule with 4 bonding pairs and 0 lone pairs?
tetrahedral with bond angles of 109.5° (e.g. CH4).
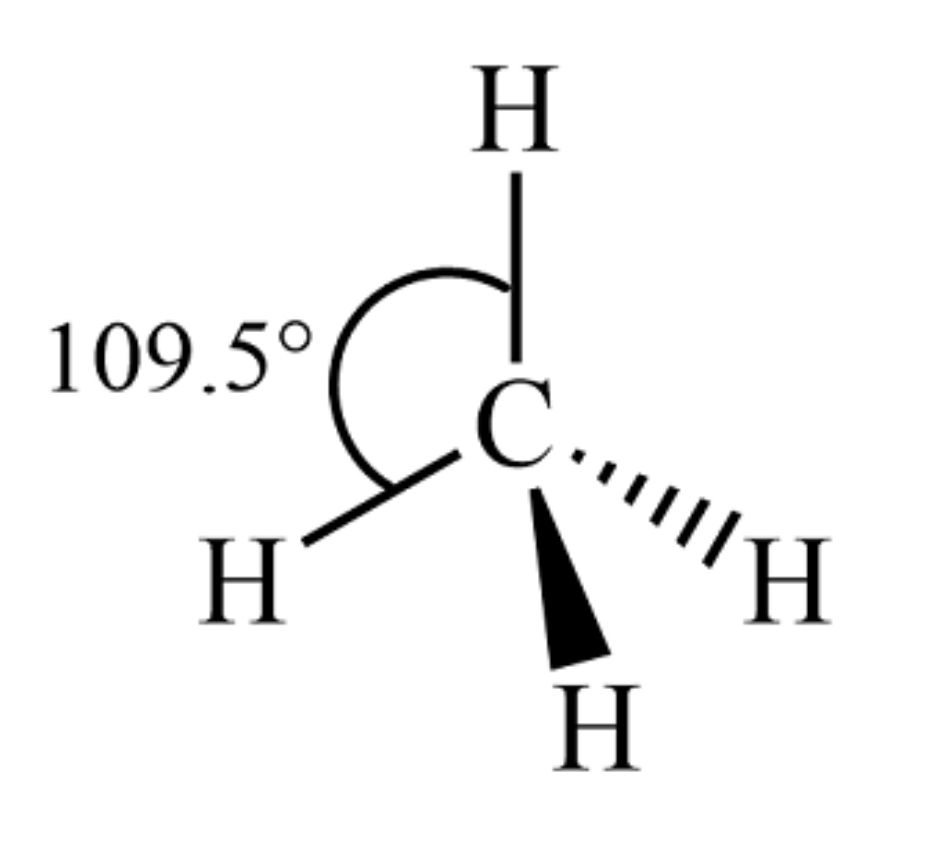
what is the shape of a molecule with 3 bonding pairs and 1 lone pair?
pyramidal with bond angles ~107° (e.g. NH3).
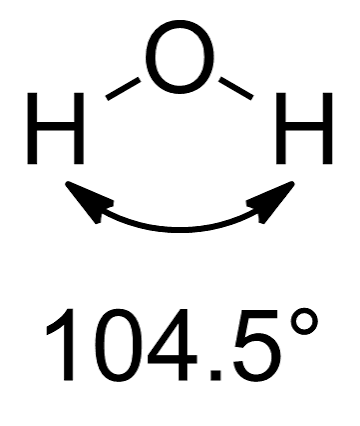
what is the shape of a molecule with 2 bonding pairs and 2 lone pairs?
non linear with bond angles ~104.5° (e.g. H2O).
what is electronegativity?
electronegativity is the ability of an atom in a molecule to attract bonding electrons towards itself.
how does electronegativity affect bond polarity?
if atoms have different electronegativities, the electrons are unequally shared, creating a polar bond (dipole).
when is a molecule polar?
a molecule is polar if it has polar bonds and the dipoles do not cancel due to asymmetrical shape (e.g. H2O).
when is a molecule non-polar?
a molecule is non-polar if the bond dipoles cancel out due to symmetrical shape (e.g. CO2, CH4), even if bonds are polar.
what is the effect of lone pairs on bond angles?
lone pairs repel more strongly than bonding pairs, which reduces bond angles compared to the ideal geometry.
why does polarity decrease down a group?
more shielding