VSEPR Bond geometry
1/51
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
52 Terms
Geometry: 2 atoms bonded to central, 0 lone pairs
Linear
Molecular Geometry: 2 atoms bonded to central, 1 lone pair
Bent 120
Molecular Geometry: 2 atoms bonded to central 2 lone pairs
Bent 109.5
Molecular Geometry: 3 atoms bonded to central, 0 lone pairs
trigonal planar
Molecular Geometry: 3 atoms bonded to central 1 lone pair
Trigonal pyramidal
Molecular Geometry: 3 atoms bonded to central 2 lone pairs
T-shaped
Molecular Geometry: 4 atoms bonded to central 0 lone pairs
Tetrahedral
Molecular Geometry: 4 atoms bonded to central 1 lone pair
See-saw
Molecular Geometry: 4 atoms bonded to central 2 lone pairs
square planar
Molecular Geometry: 5 atoms bonded to central 0 lone pairs
trigonal bipyramidal
Molecular Geometry: 5 atoms bonded to central 1 lone pair
square pyramid
Molecular Geometry: 6 atoms bonded to central 0 lone pairs
Octahedron
Bond Angle: Linear
180
Bond Angle: Bent 120
<120
Bond angle: Bent 109.5
«109.5
Bond Angle: Trigonal Planar
120
Bond Angle: tetrahedral
109.5
Molecular Geometry:2 atoms bonded to central 3 lone pairs
Linear
Bond Angle: Trigonal Pyramidal
<109.5
Bond Angle: Trigonal Bipyramidal
90,120,180
Bond Angle: Seesaw
90,120,180
Bond Angle: T-shaped
90,180
Bond Angle: Octahedral
90, 180
Bond angle: Square Pyramidal
90
Bond angle: Square planar
90
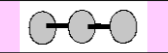
Electronic Geometry:
Linear
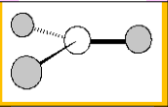
Electronic Geometry
Trigonal Planar
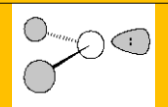
Electronic Geometry:
Trigonal Planar
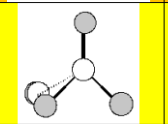
Electronic Geometry:
tetrahedral
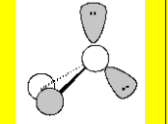
Electronic Geometry
Tetrahedral
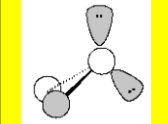
Electronic Geometry
Tetrahedral
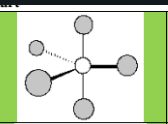
Electronic Geometry
Trigonal Bipyramidal
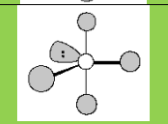
Electronic Geometry:
Trigonal Bipyramidal
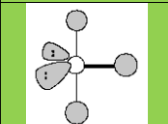
Electronic geometry
Trigonal Bipyramidal
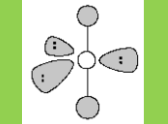
Electronic Geometry
Trigonal Bipyramidal
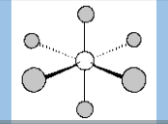
Electronic Geometry
Octahedral
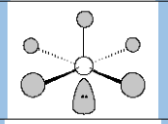
Electronic Geometry
Octahedral
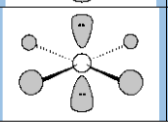
Electronic Geometry
Octahedral
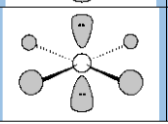
Molecular Geometry
Square planar
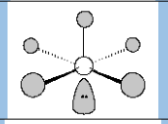
Molecular geometry
square pyramidal
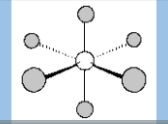
Molecular Geometry
Octahedral
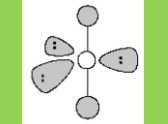
Molecular Geometry
Trigonal Bipyramidal
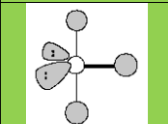
Molecular geometry
T-shaped
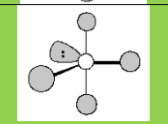
Molecular Geometry
See-saw
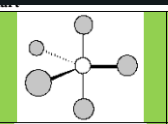
Molecular Geometry
Trigonal Bipyramidal
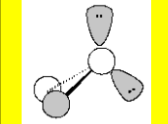
Molecular Geometry
Bent 109.5
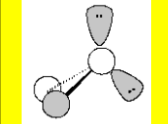
Molecular Geometry
Trigonal Pyramidal
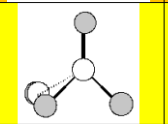
Molecular Geometry
Tetrahedral
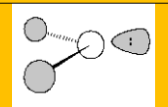
Molecular Geometry
Bent 120
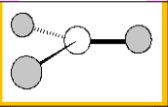
Molecular Geometry
Bent 120
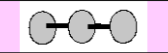
Molecular Geometry
Linear