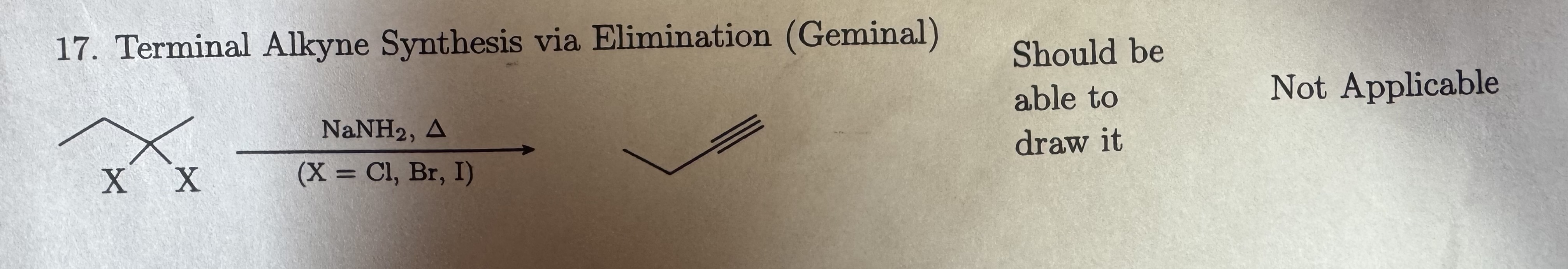Alkynes Reactions
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
17 Terms
HX
X = Cl, Br
Hydrohalogenation of Alkynes
Produce Vinyl Dihalide
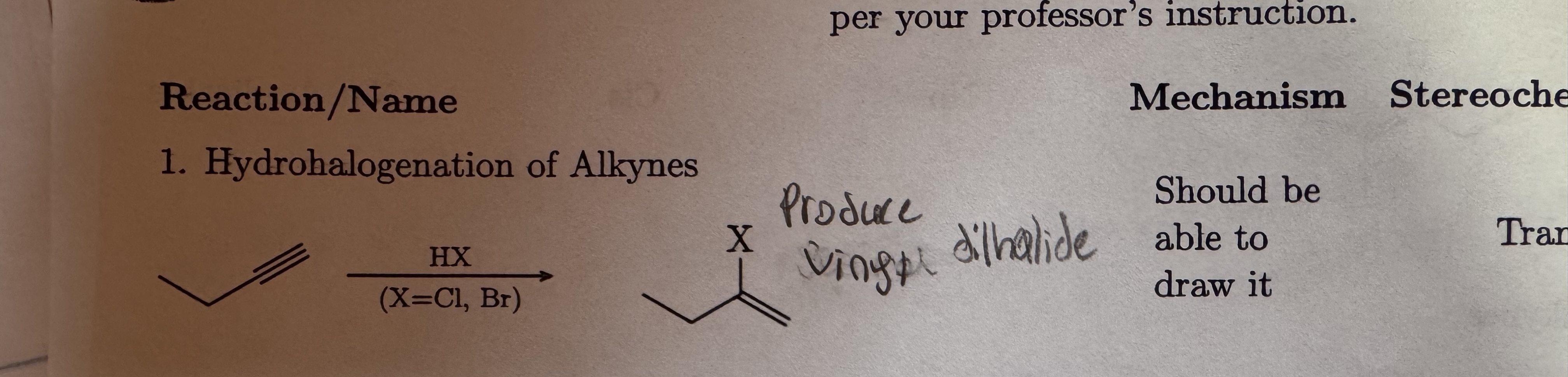
2 equation HX
X = Cl, Br
Hydrohalogenation of Alkynes (2 equations) Geminal Dihalides
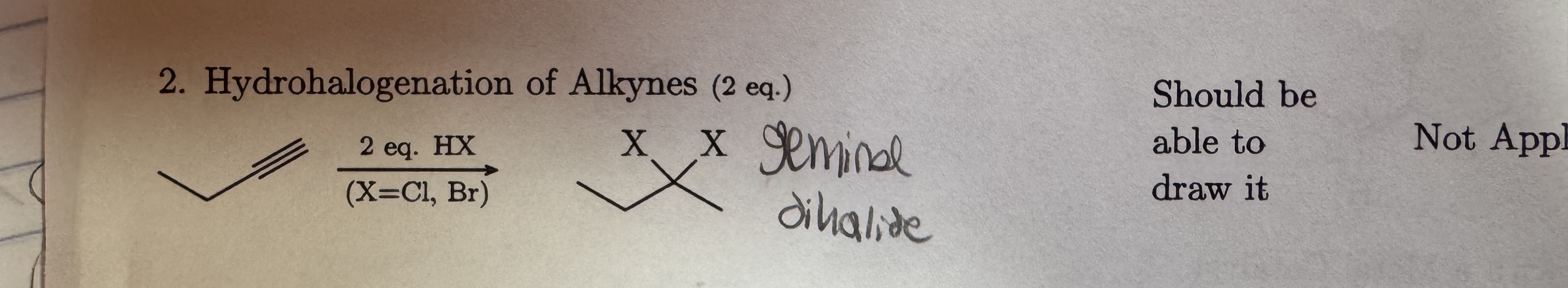
X2 = CH2Cl2
(X2 = Cl2, Br2)
Halogenation of Alkynes (trans-dihalide)

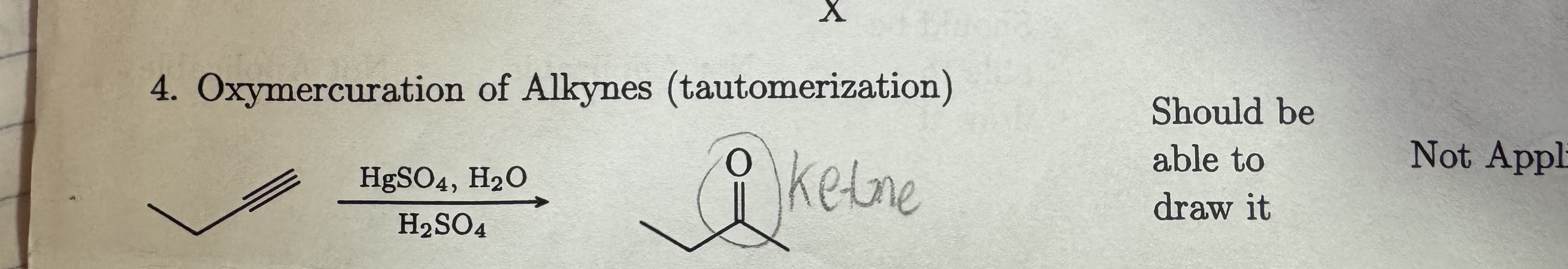
HgSO4, H2O
H2SO4
Oxymercuration of Alkynes
with a ketone
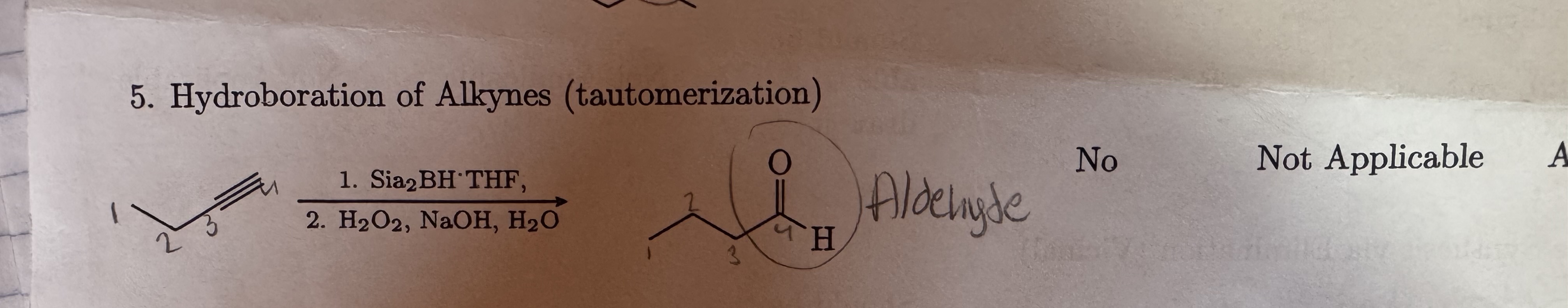
Sia2BH • THF
H2O2, NaOH, H2O
Hydroboration of Alkynes
Aldehydes addition
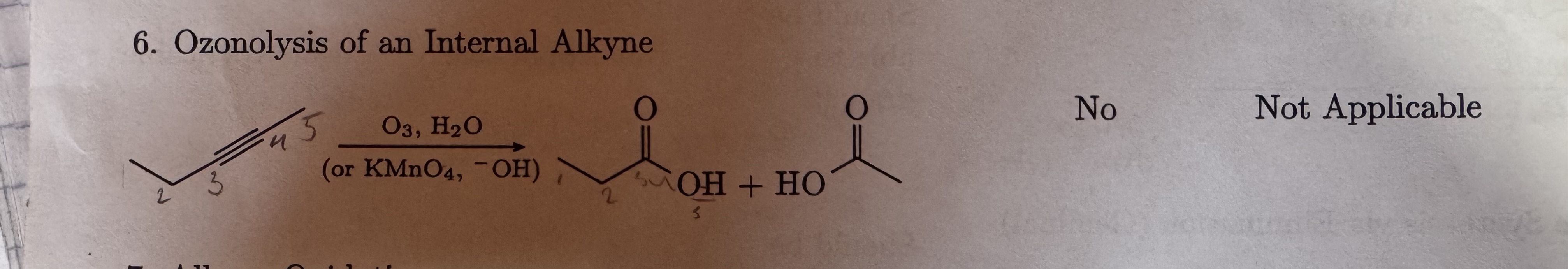
O3, H2O
or KMnO4, -OH
Ozonolysis of an Internal Alkyne
Carboxylic acid
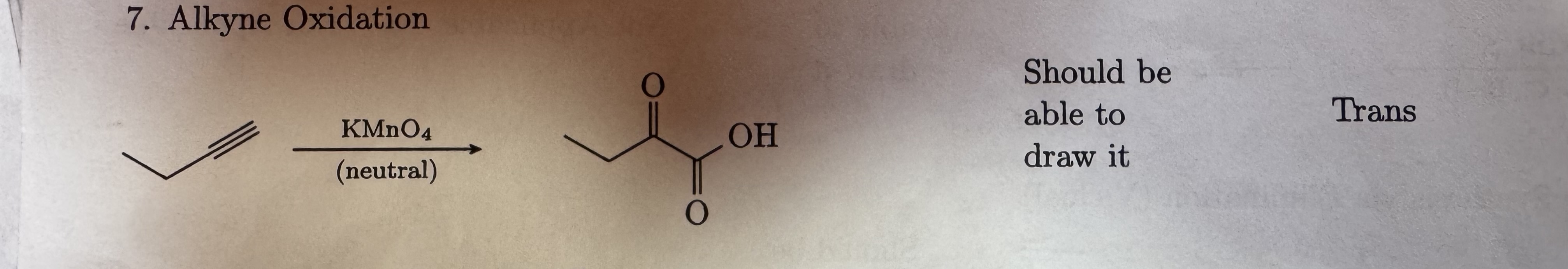
KMnO4
neutral
Alkyne Oxidation
Trans -OH and 2 ketones
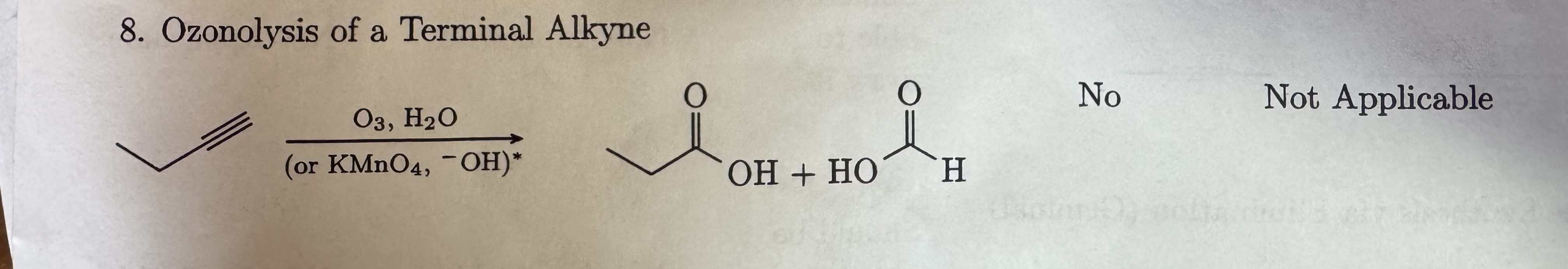
O3, H2O
or KMnO4, -OH terminal
Ozonolysis of terminal alkyne
carboxylic acid
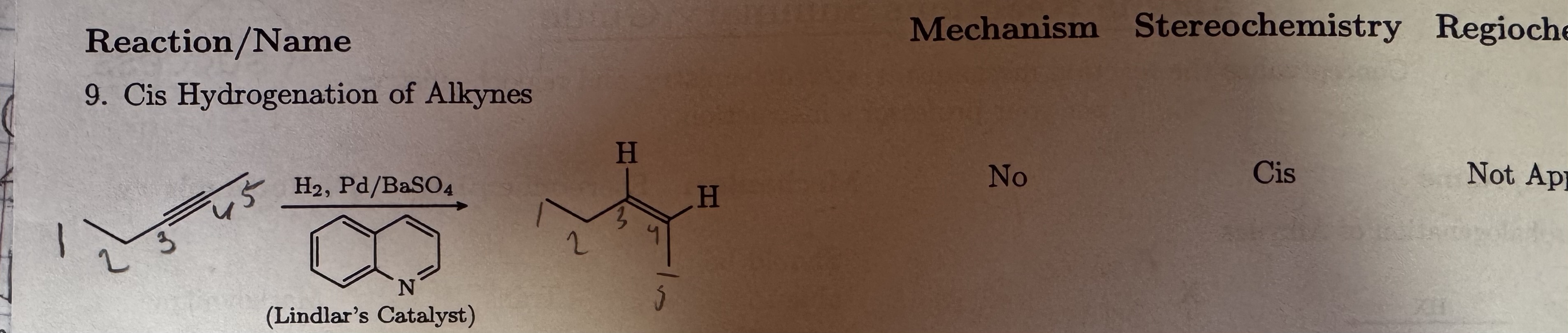
H2, Pd/BaSO4
Lindlars Catalyst
Cis Hydrogenation of Alkynes
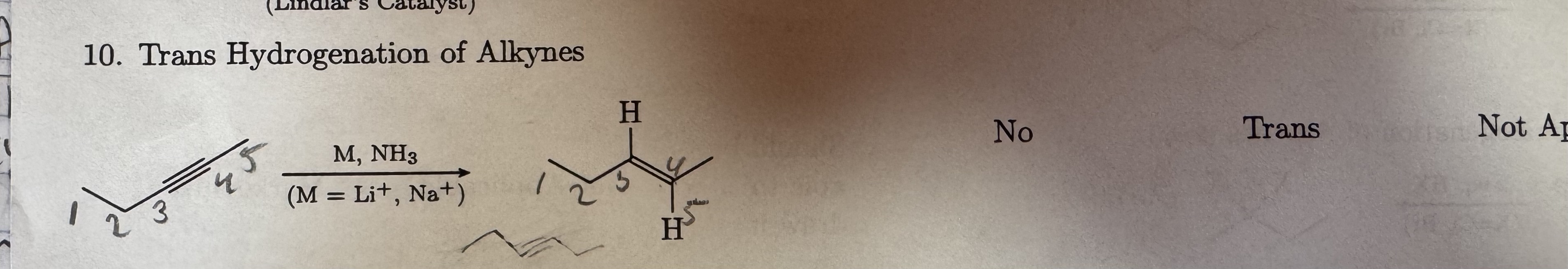
M, NH3
M = Li+, Na+
Trans Hydrogenation of Alkynes
H2, Pd
Hydrogenation of Alkynes with a Metal Catalyst (2 Hydrogens on 2 carbons each)

NaNH2
Deprotonation of Alkynes
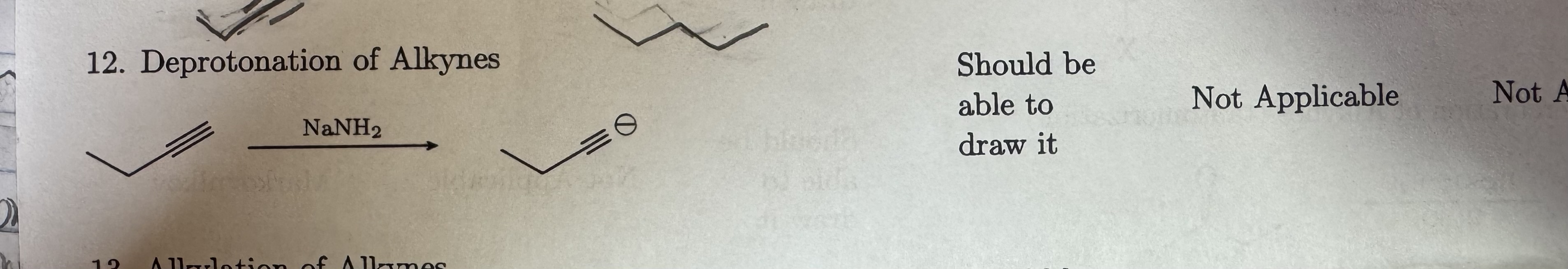
NaNH2
RX
Alkylation of Alkynes
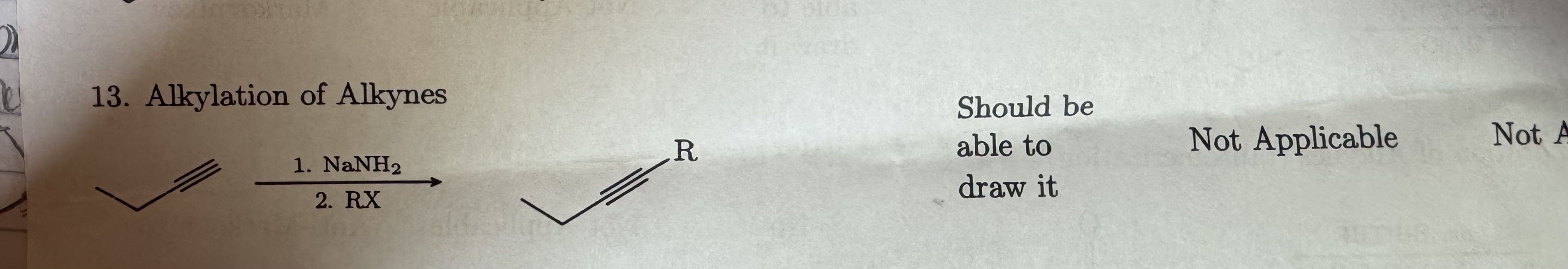
KOH, heat symbol
X = Cl, Br, I for Vicinal
Internal Alkyne Synthesis via Elimination (Vicinal)
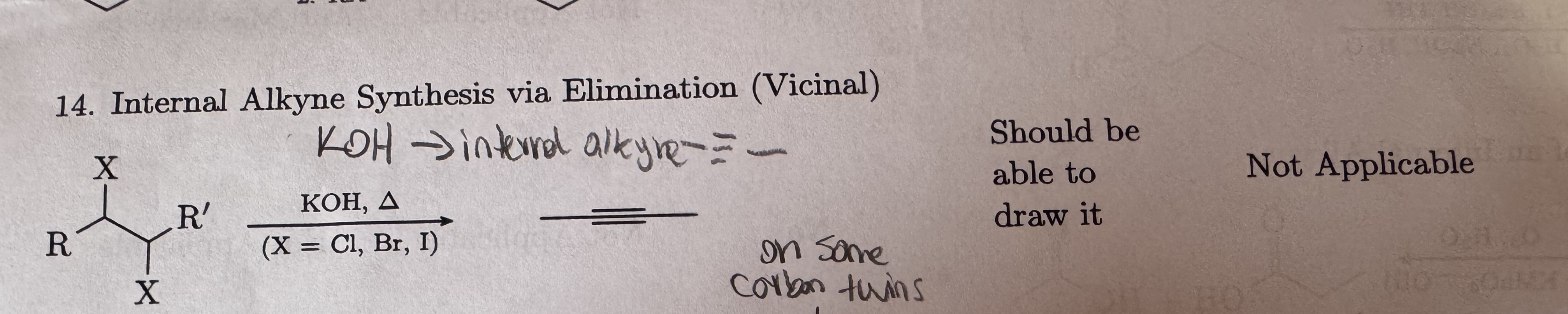
KOH, heat symbol
X = Cl, Br, I for Geminal
Internal Alkyne Synthesis via Elimination (Geminal)

NaNH2, heat symbol
(X = Cl, Br, I) (Vicinal)
Internal Alkyne Synthesis via Elimination (Vicinal)
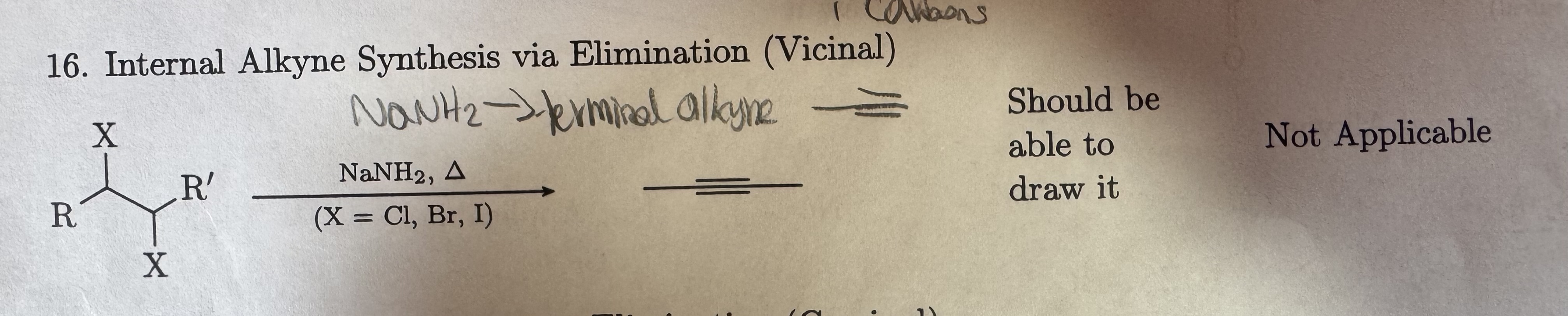
NaNH2, heat symbol
X = Cl, Br, I (Geminal)
Terminal Alkyne Synthesis via Elimination (Geminal)