CHEM 2110 / Topic 5b: Carboxylic Acid Derivatives
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
29 Terms
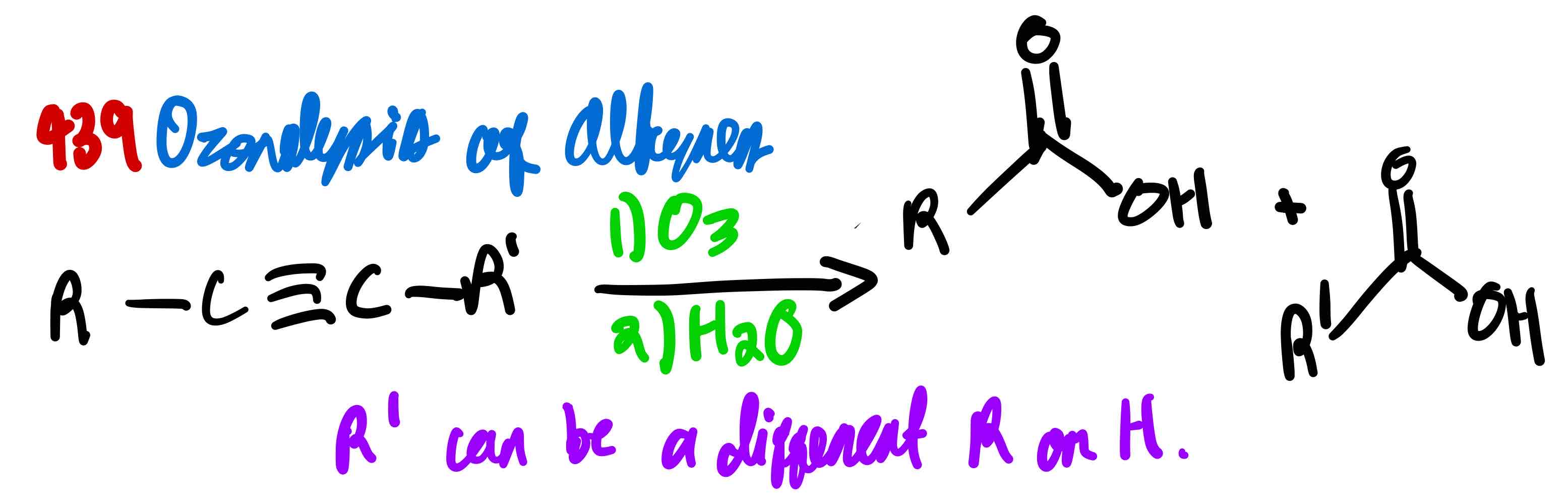
From alkyne to carboxylic acid, how?
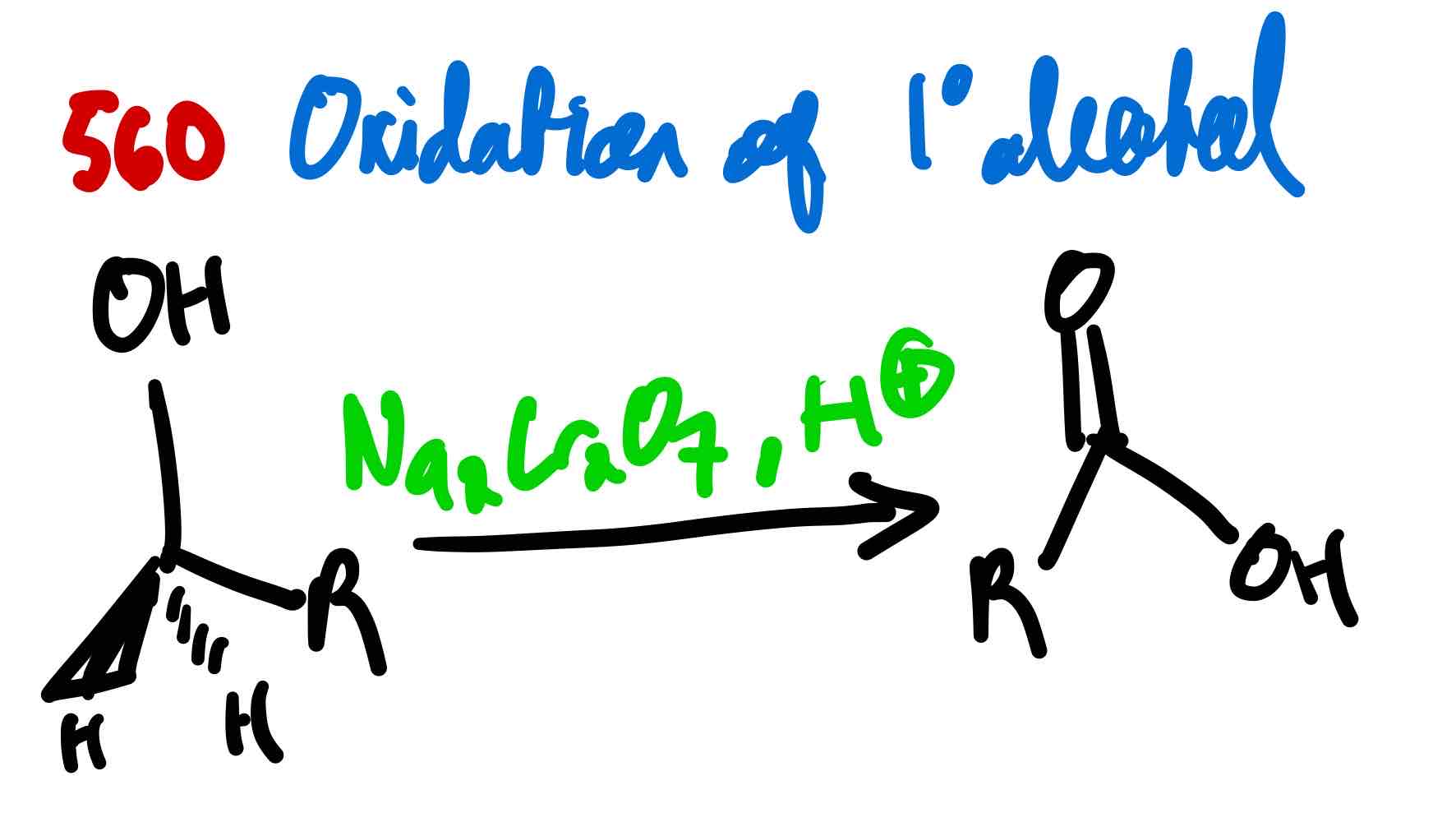
From 1º alcohol to carboxylic acid, how?
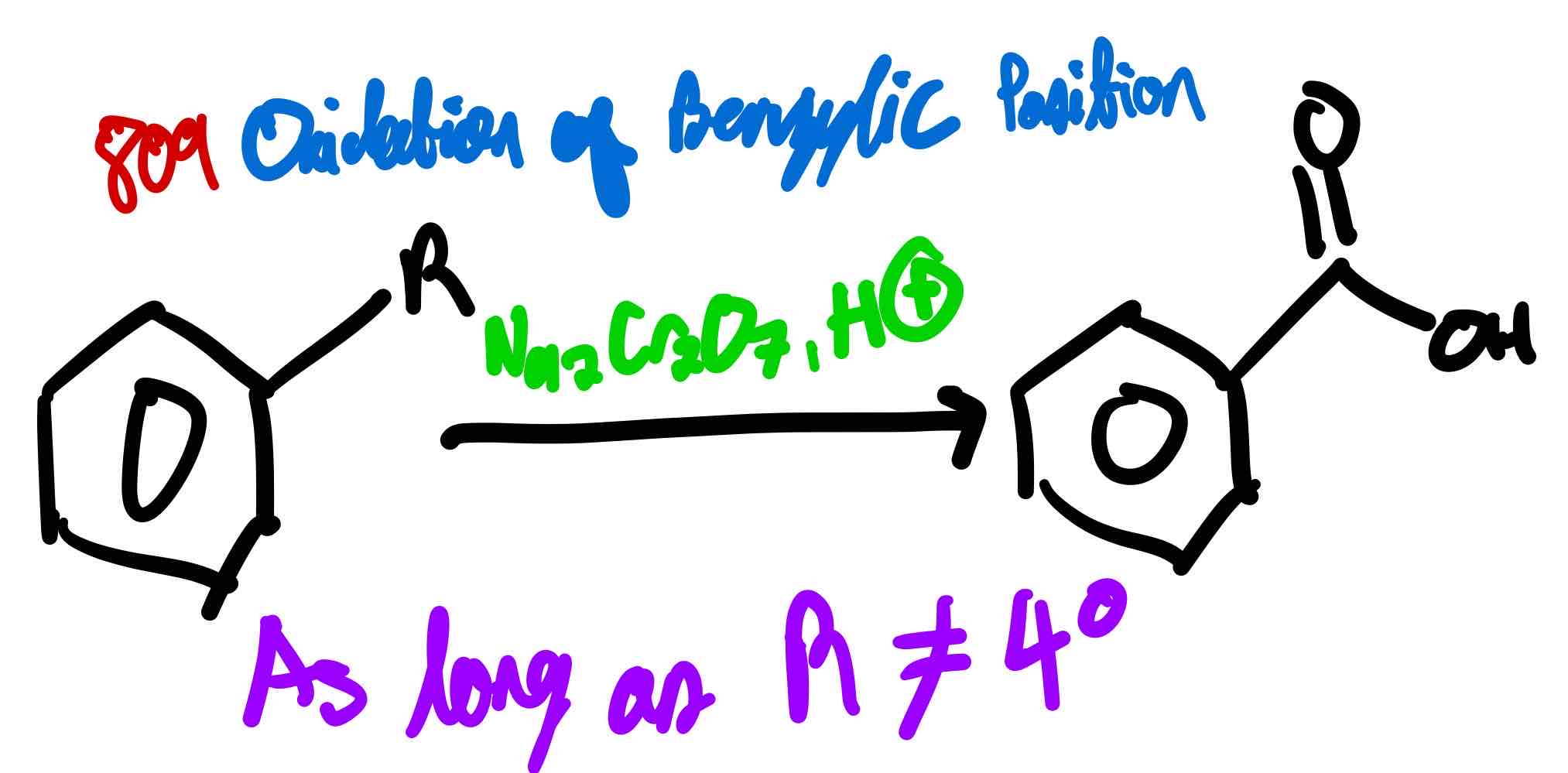
From benzene with R group at benzylic position to benzoic acid, how?
If you only had a Grignard reagent, how can you make a carboxylic acid? Furthermore, if you had an alkyl halide, how can you make the same thing?
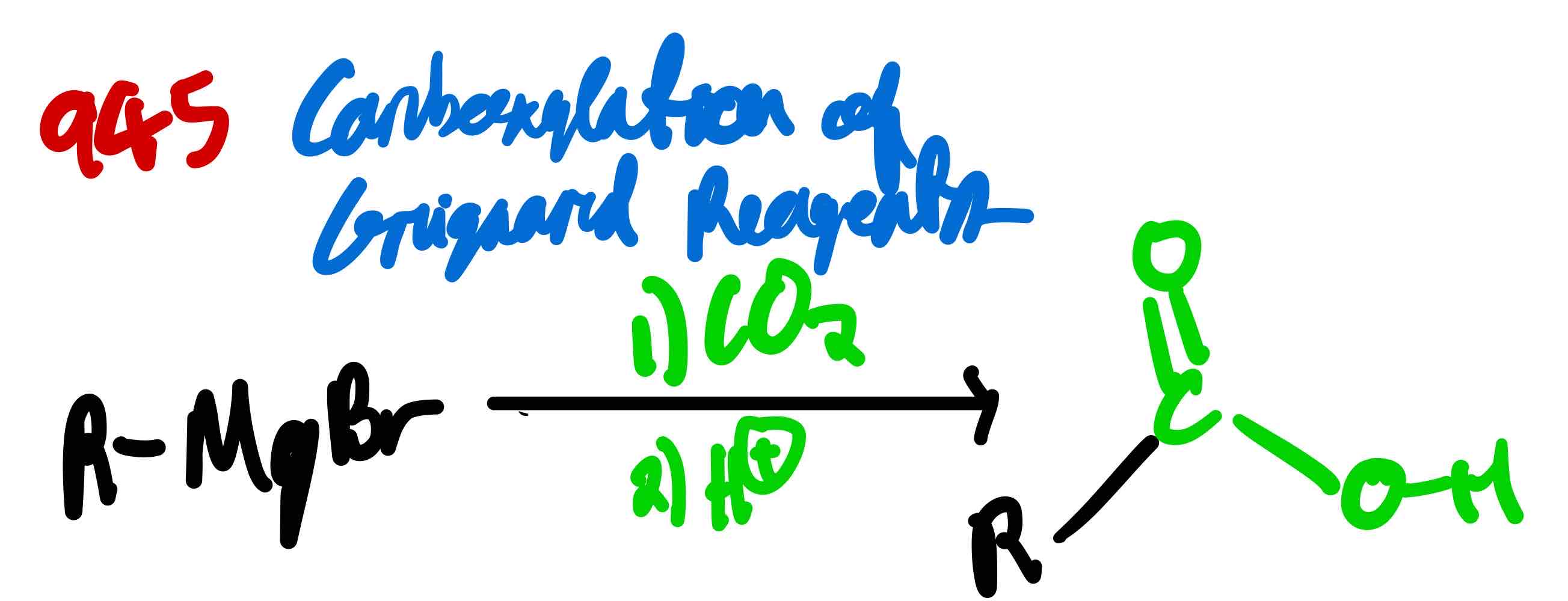
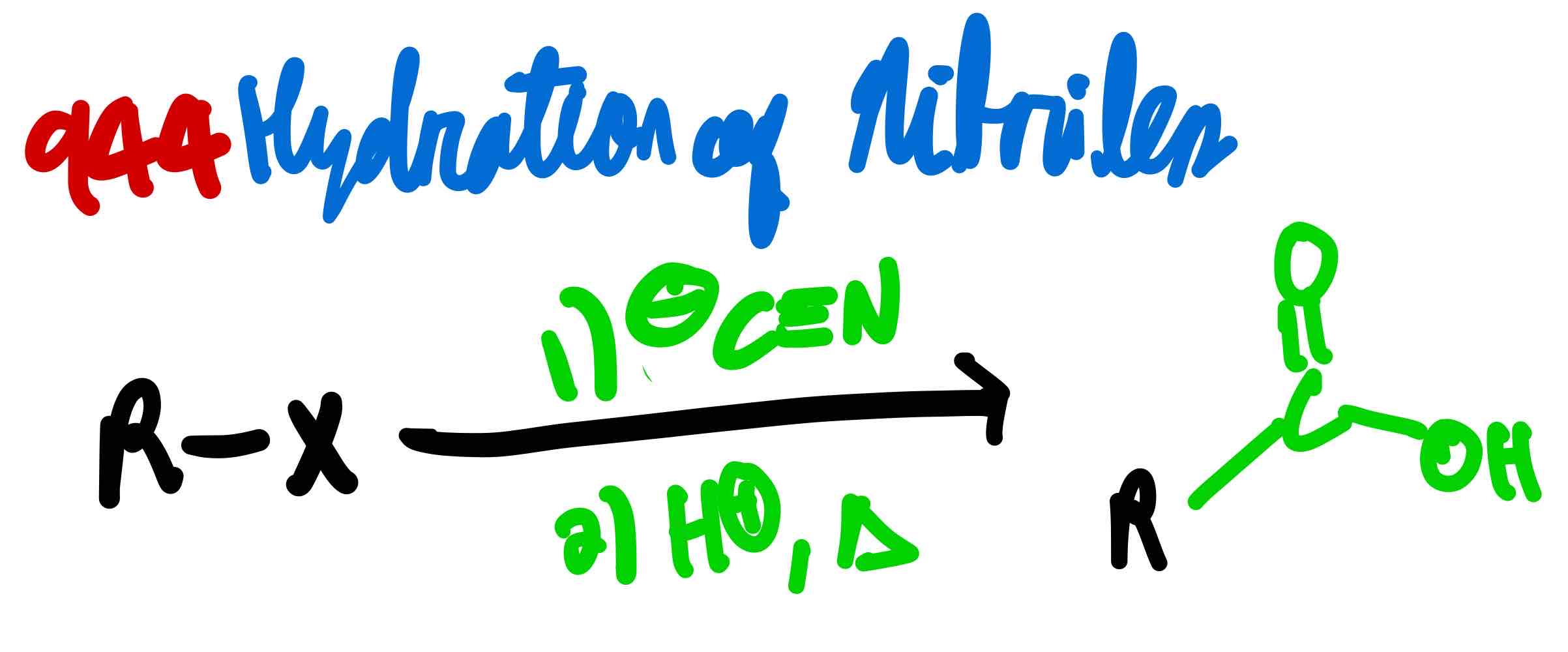
If you had a methyl or primary alkyl halide, how can you make a carboxylic acid?
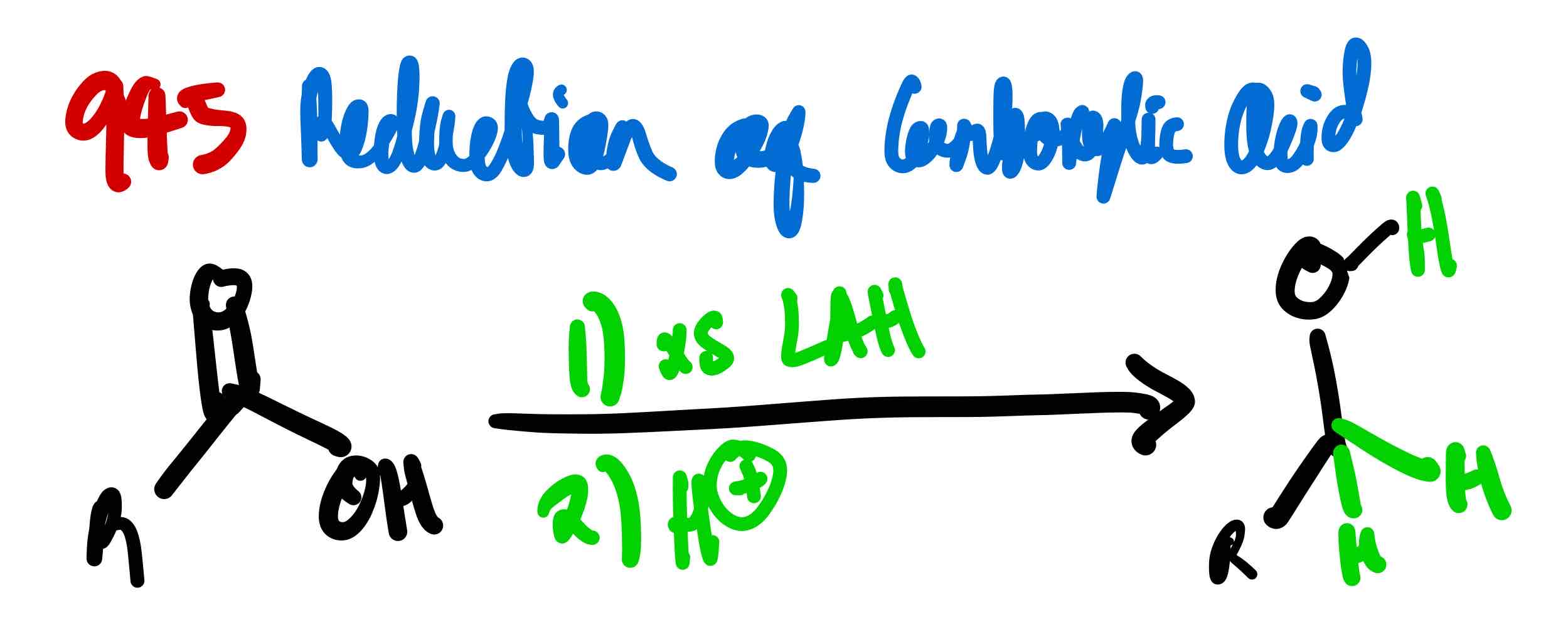
If you wanted to turn a carboxylic acid to a 1º alcohol, how would you proceed?
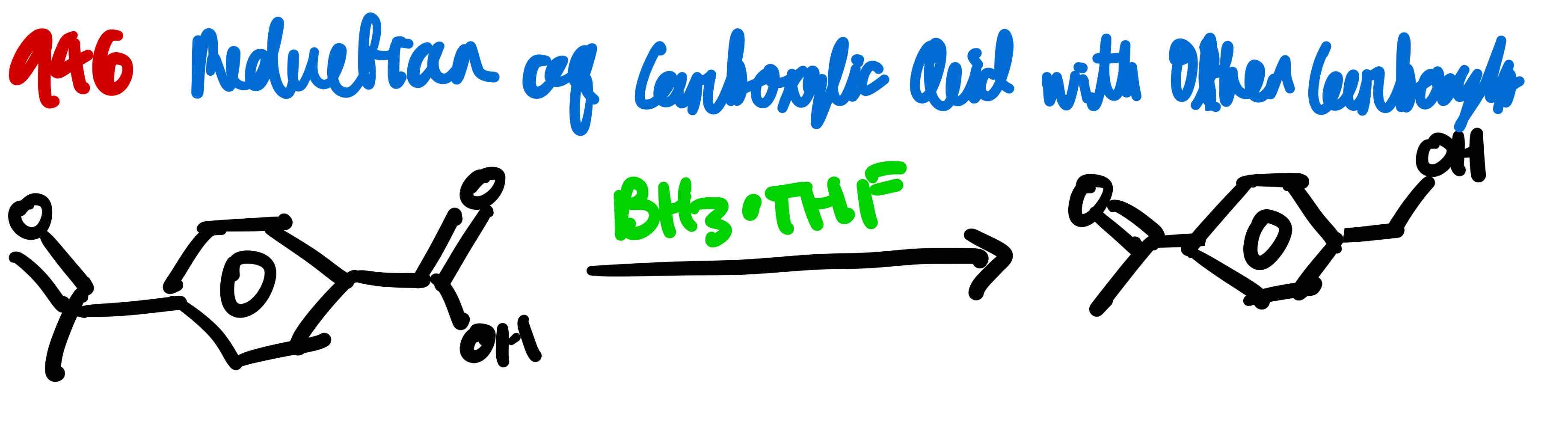
If you had another C=O present in a molecule that also had C=O but in a carboxylic acid, how would you turn the C=O of the carboxylic acid to a 1º alcohol?
From carboxylic acid to acid chloride, how?

From acid chloride to carboxylic acid, how?
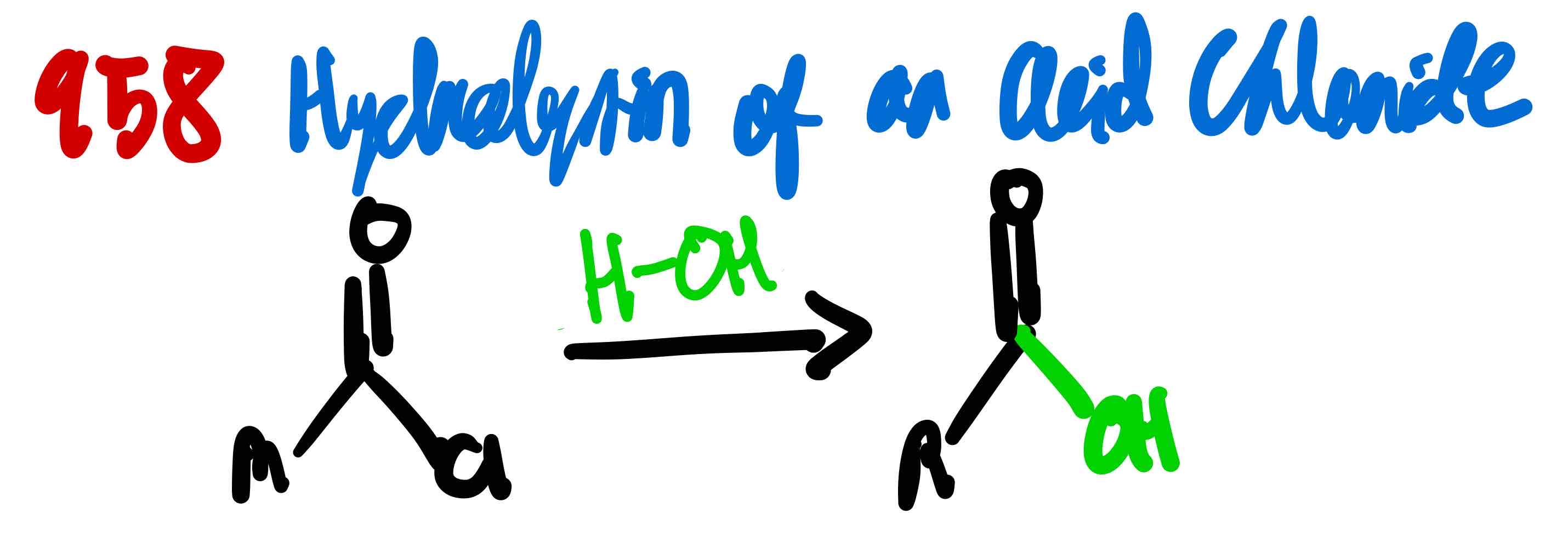
From acid chloride to 1º alcohol, how?
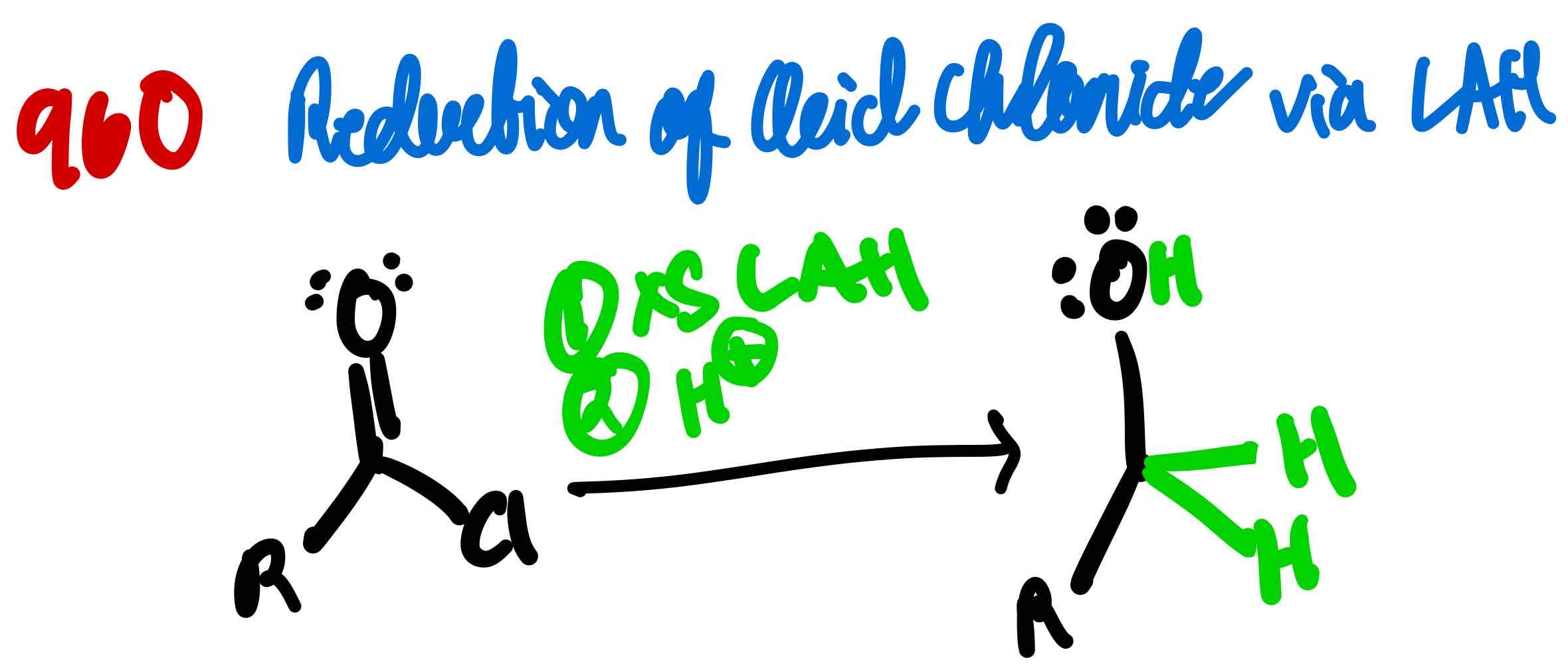
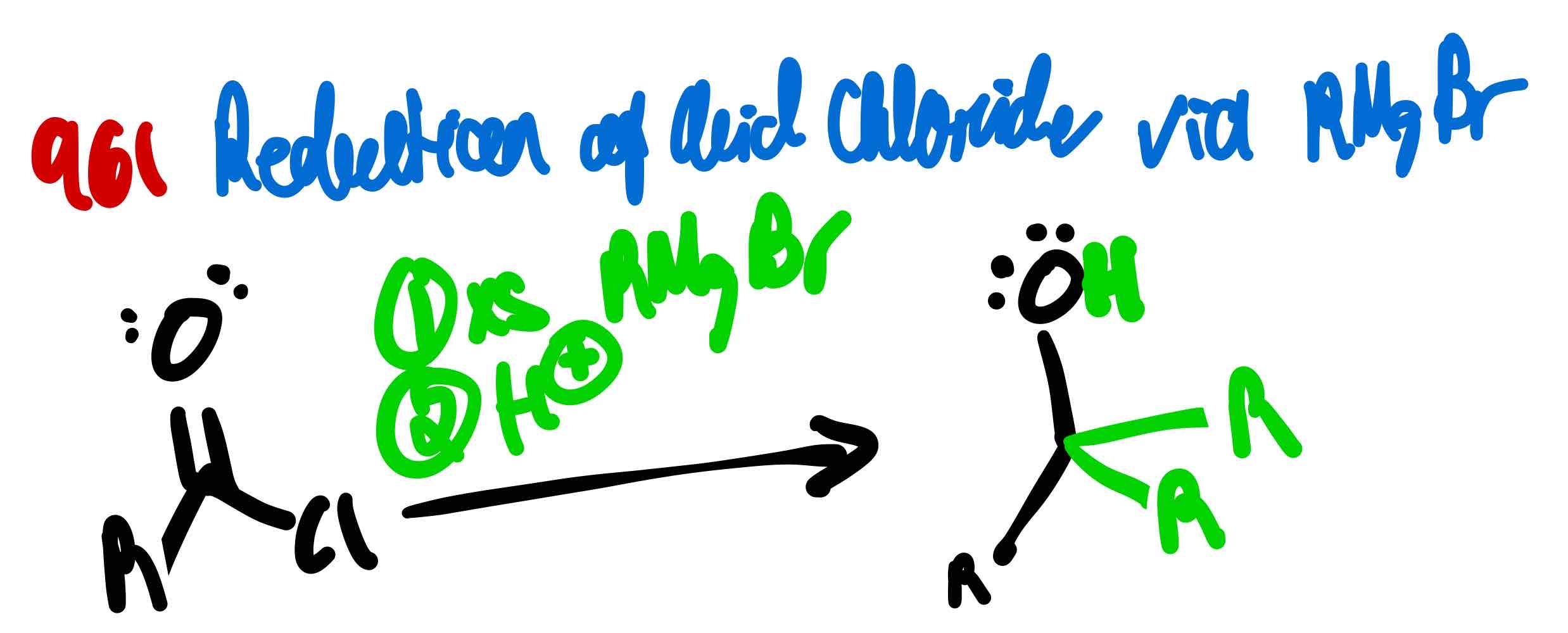
From acid chloride to 3º alcohol, how?
From acid chloride to aldehyde, how?

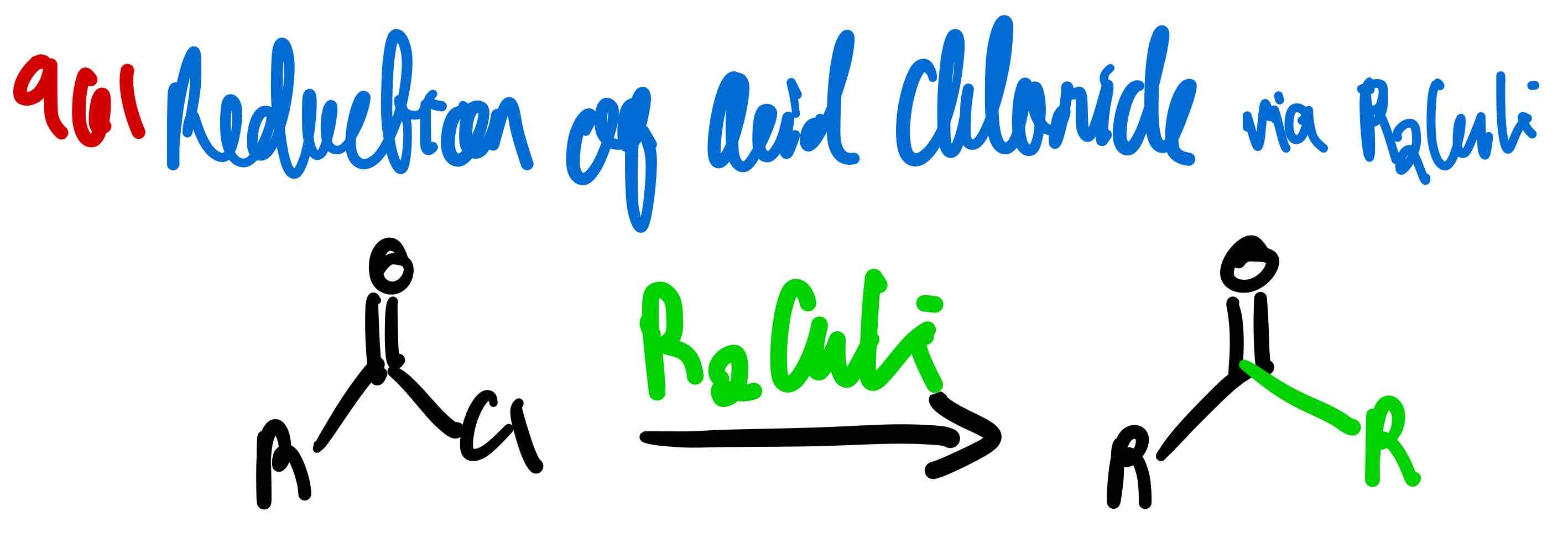
From acid chloride to ketone, how?
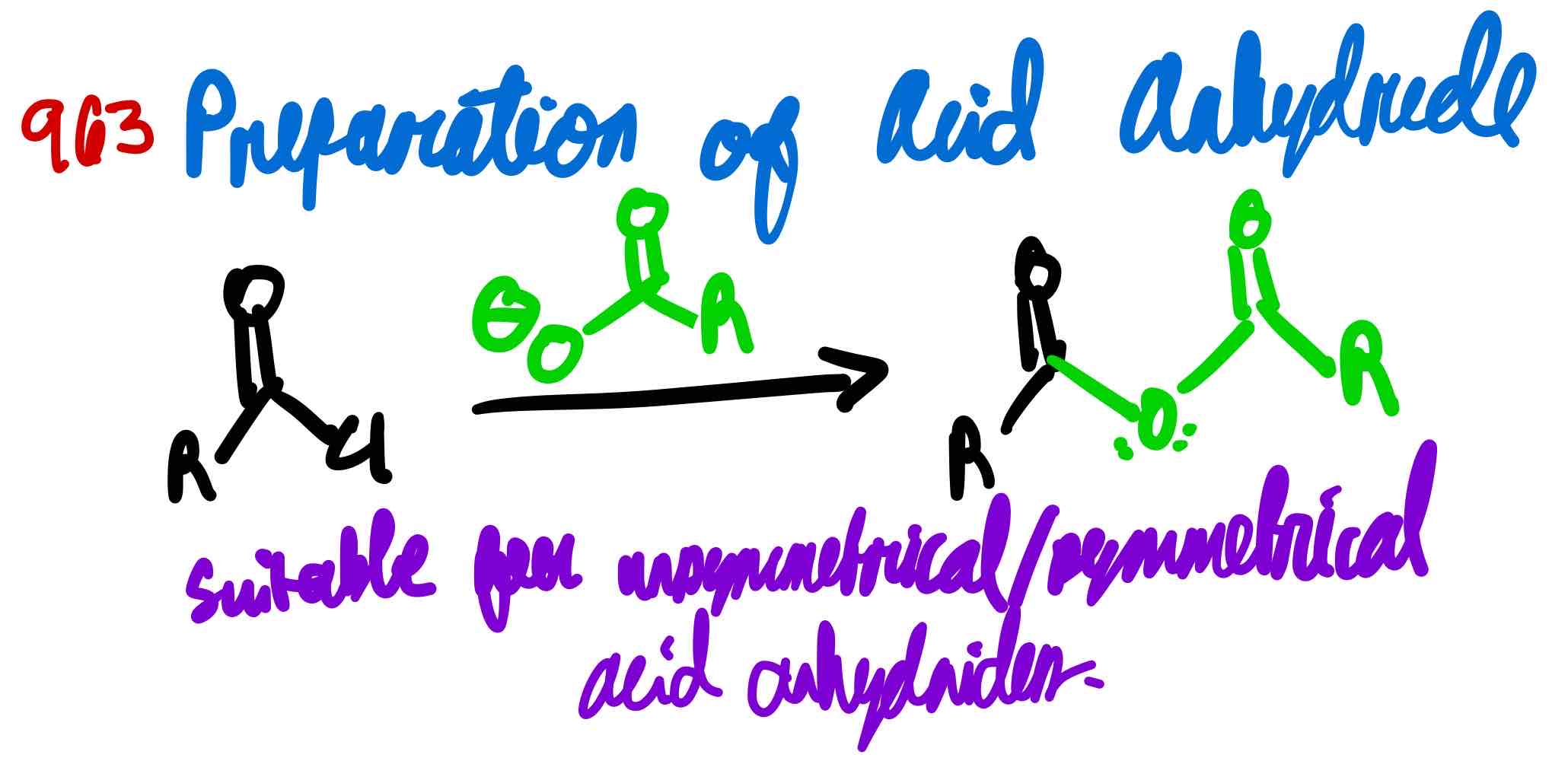
From acid chloride to acid anhydride, how?
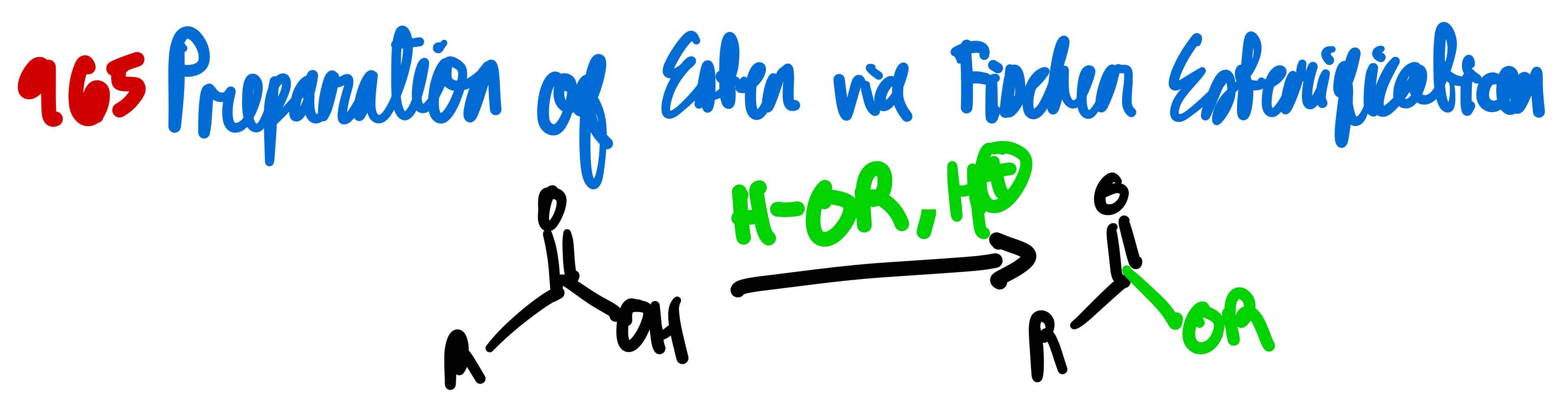
From carboxylic acid to ester, how?
From acid chloride to ester, how?
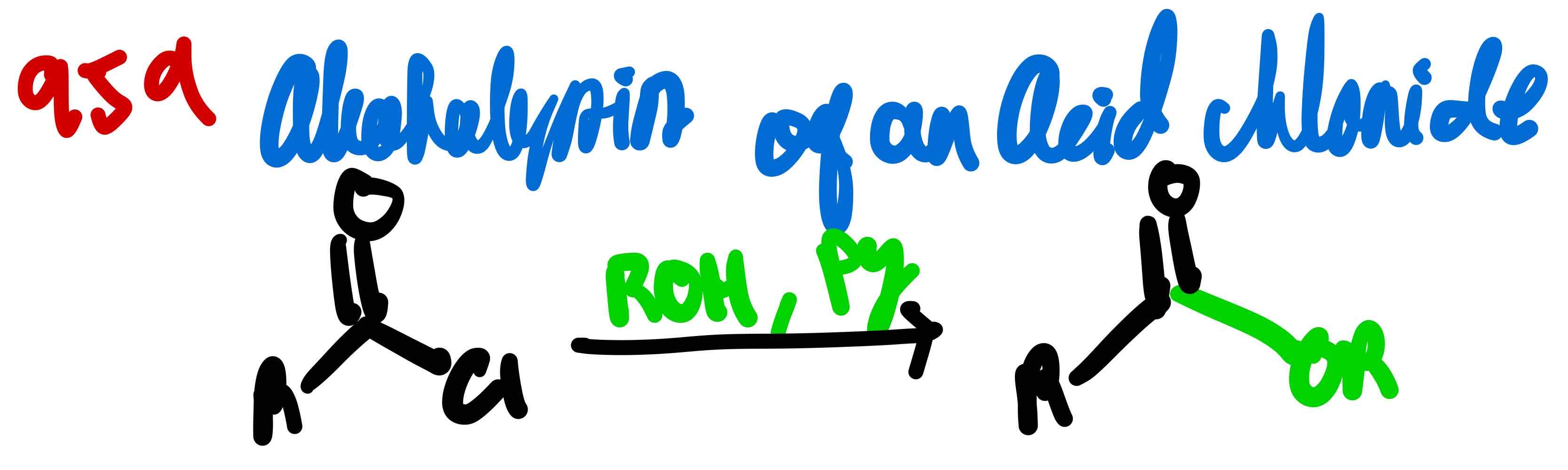
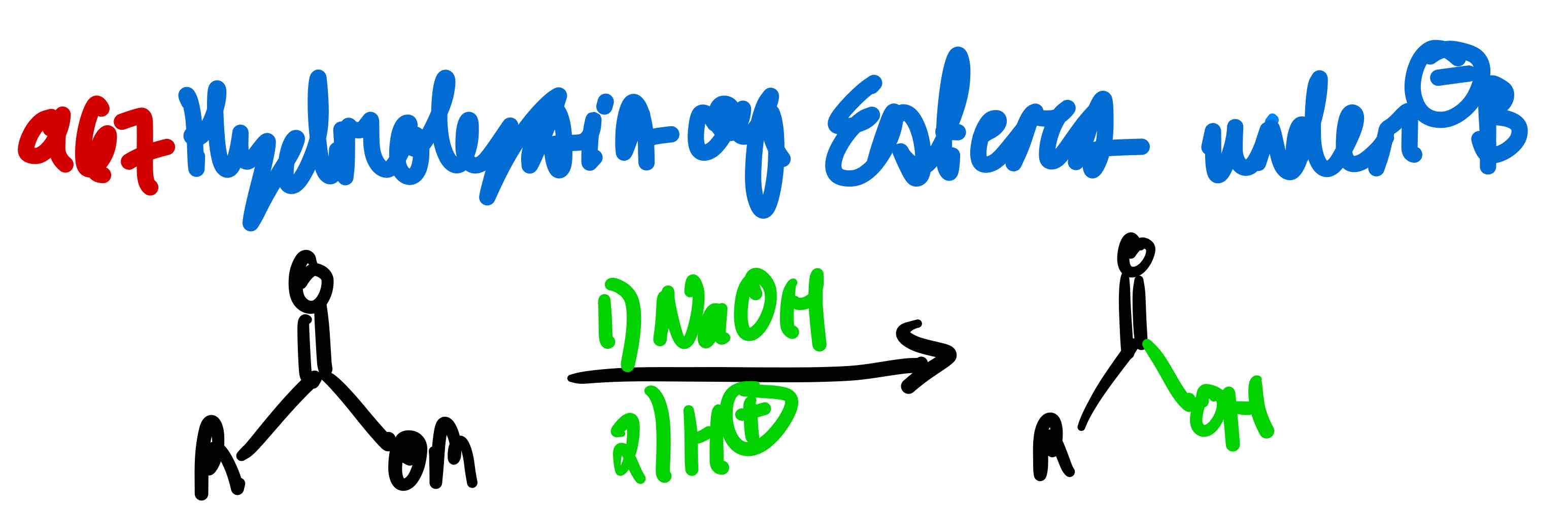
From ester to carboxylic acid in basic conditions, how?
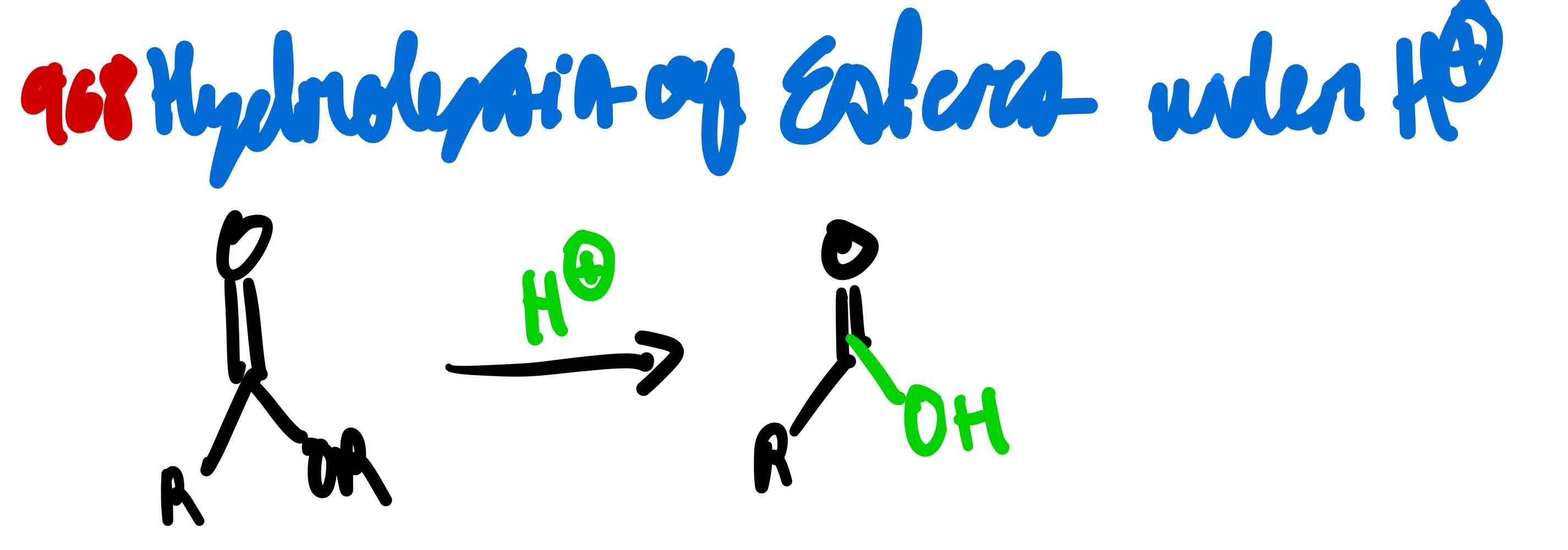
From ester to carboxylic acid in acidic conditions, how?
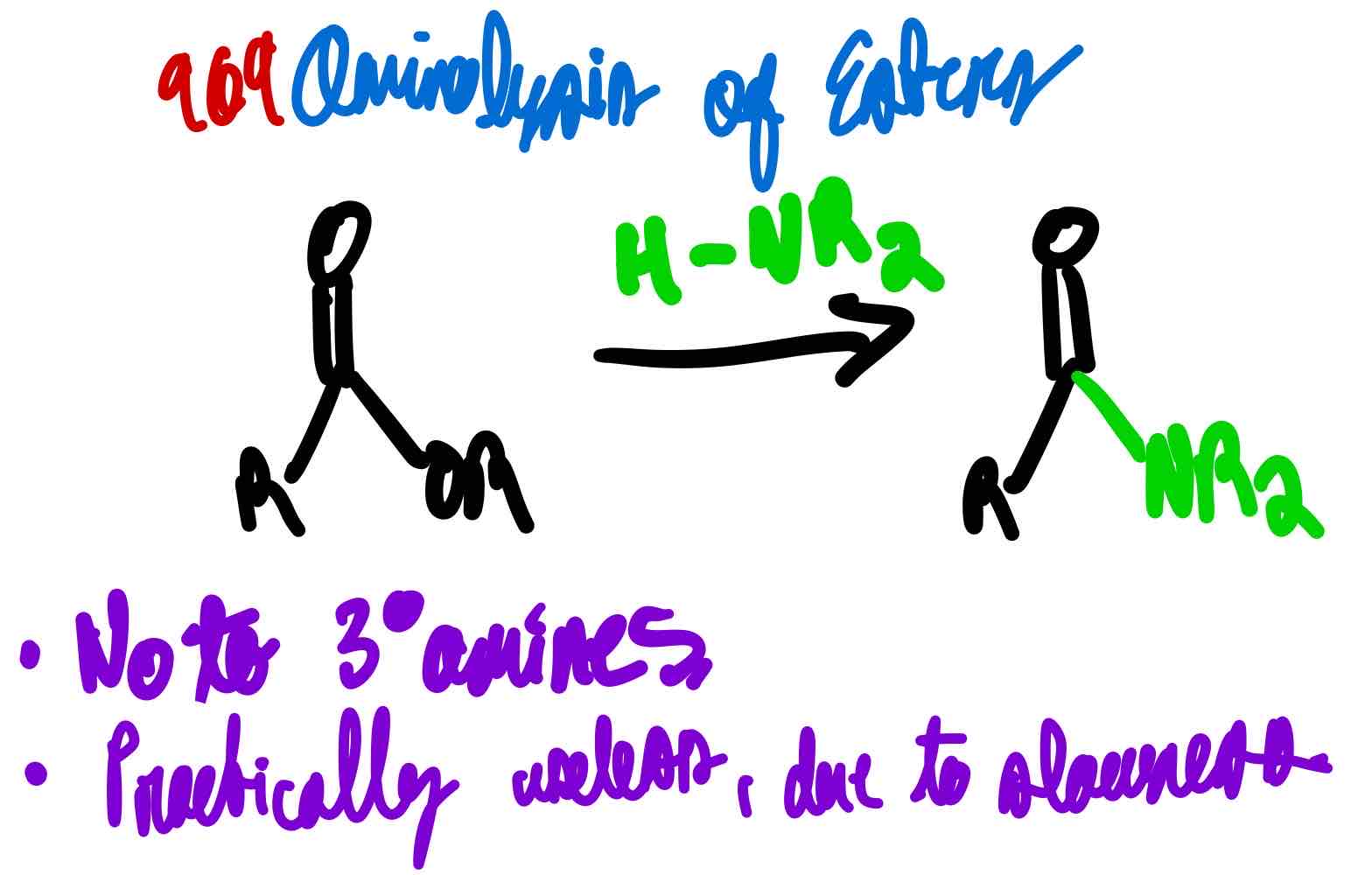
From ester to amide, how?
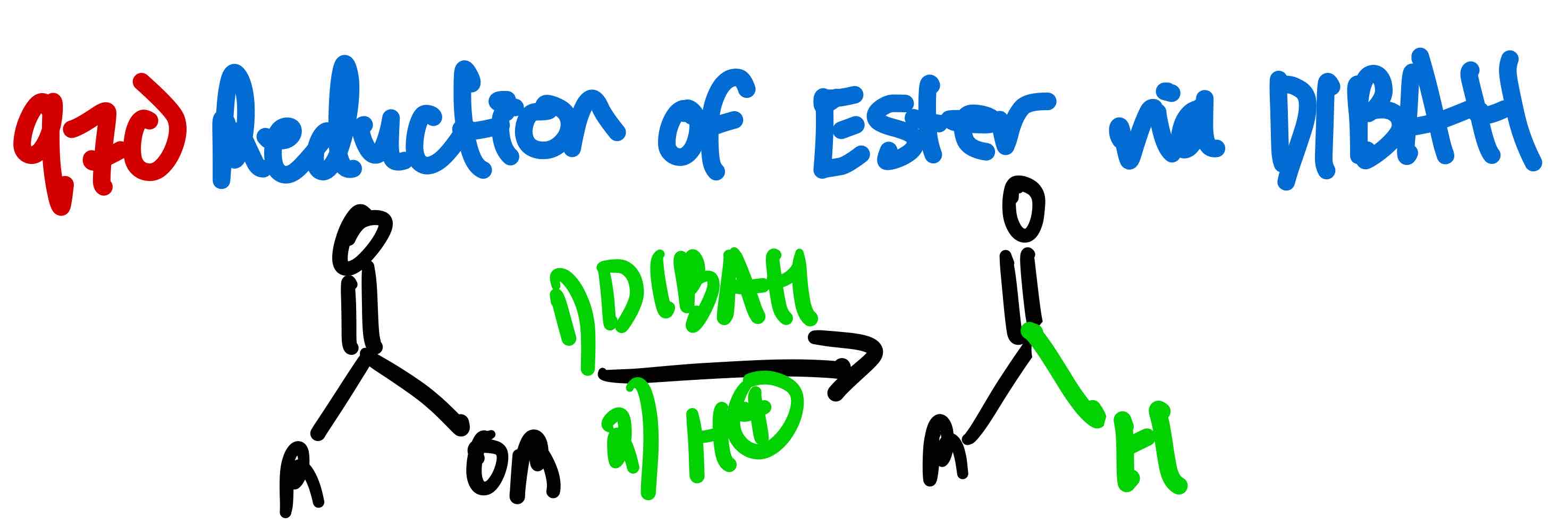
From ester to aldehyde, how?
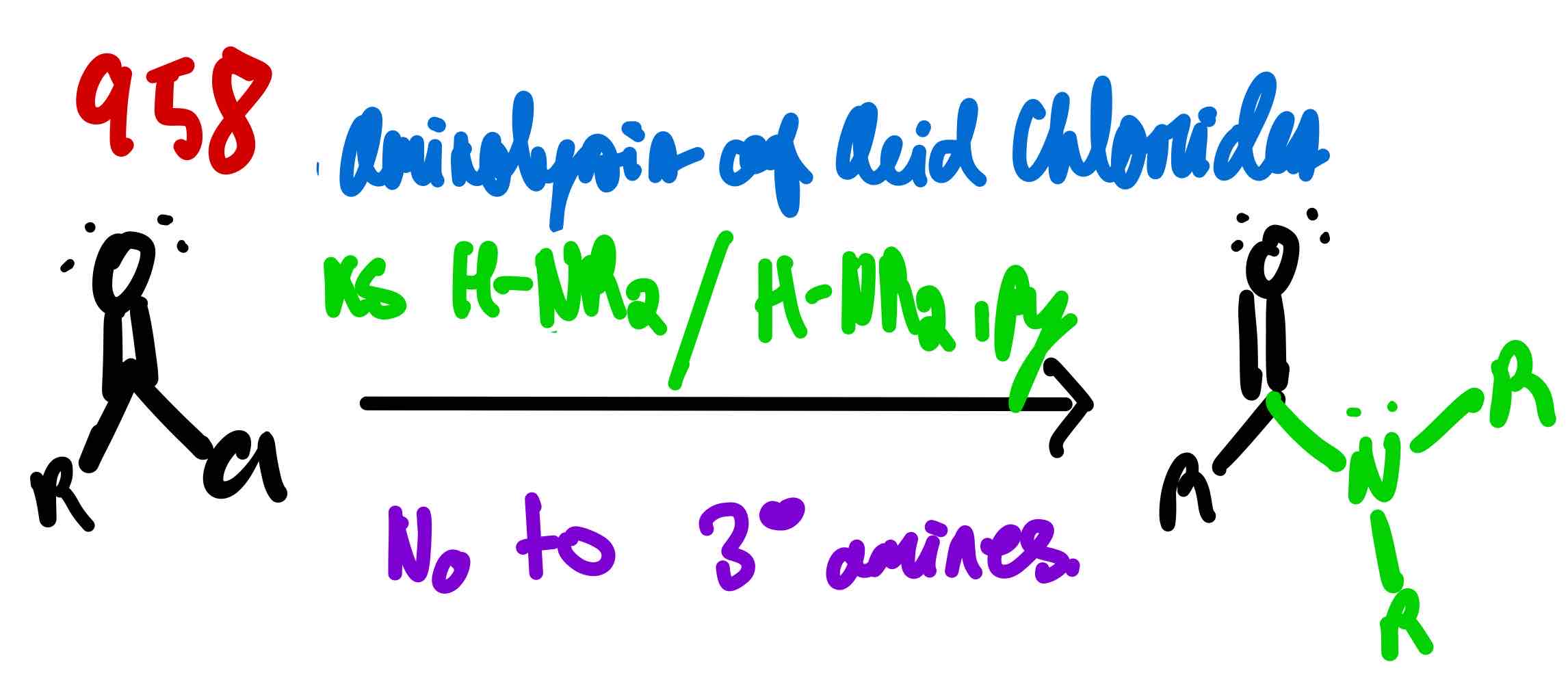
From acid chloride to amide, how?
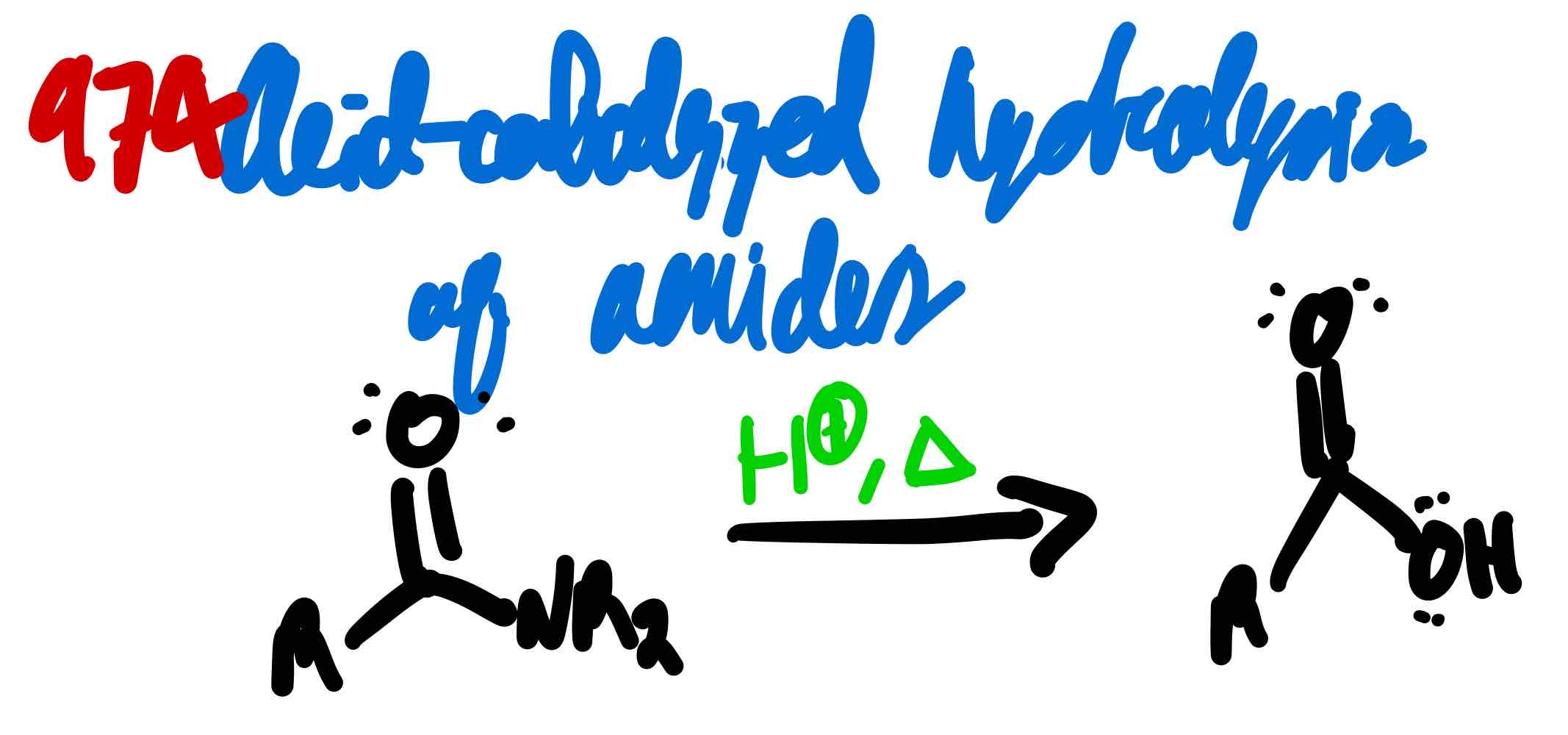
From amide to carboxylic acid in acidic conditions, how?
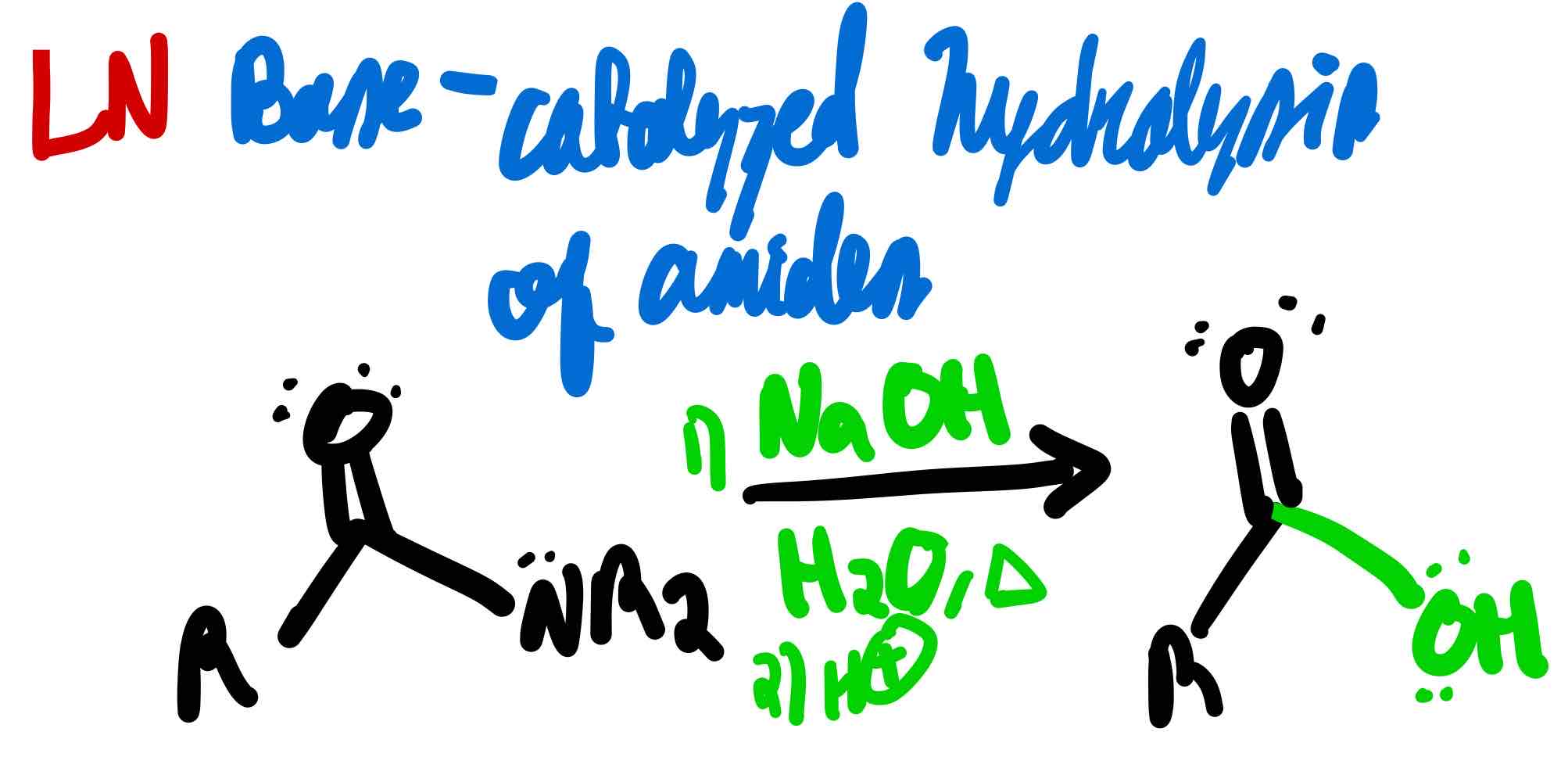
From amide to carboxylic acid in basic conditions, how?
From amide to amine, how?
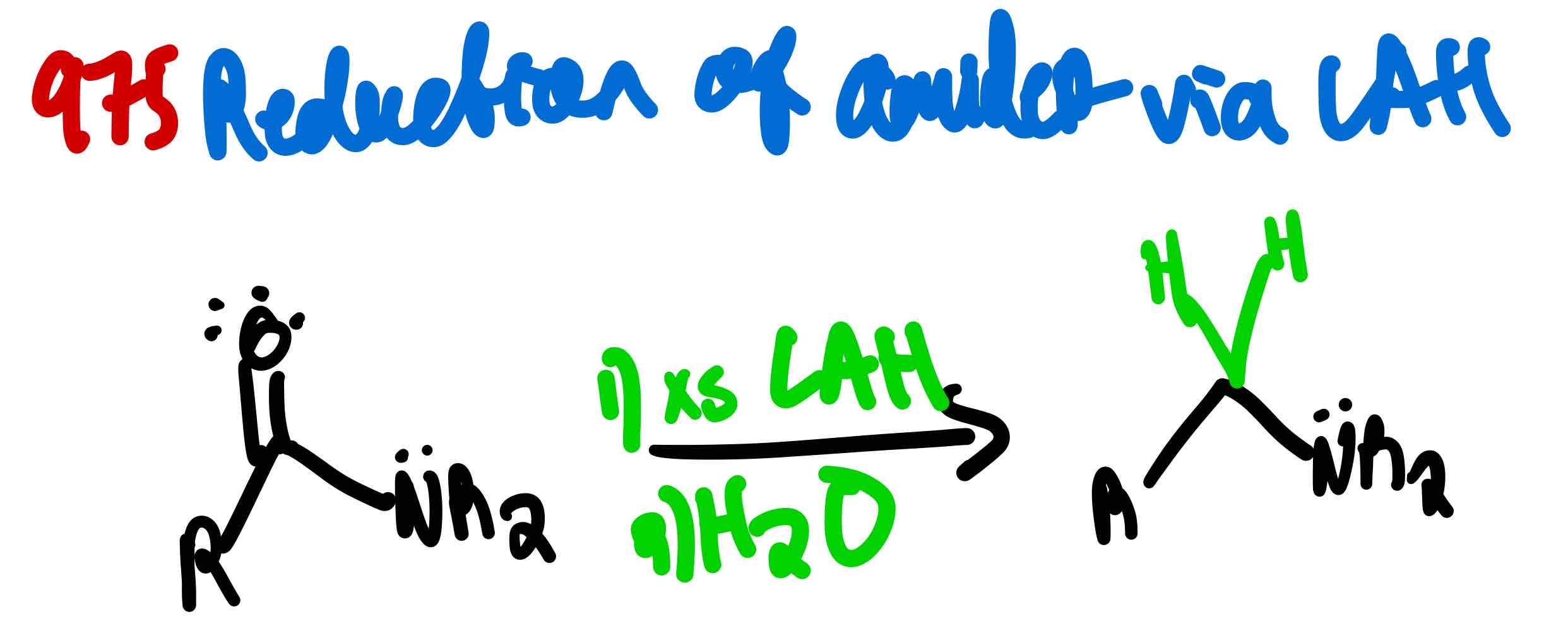
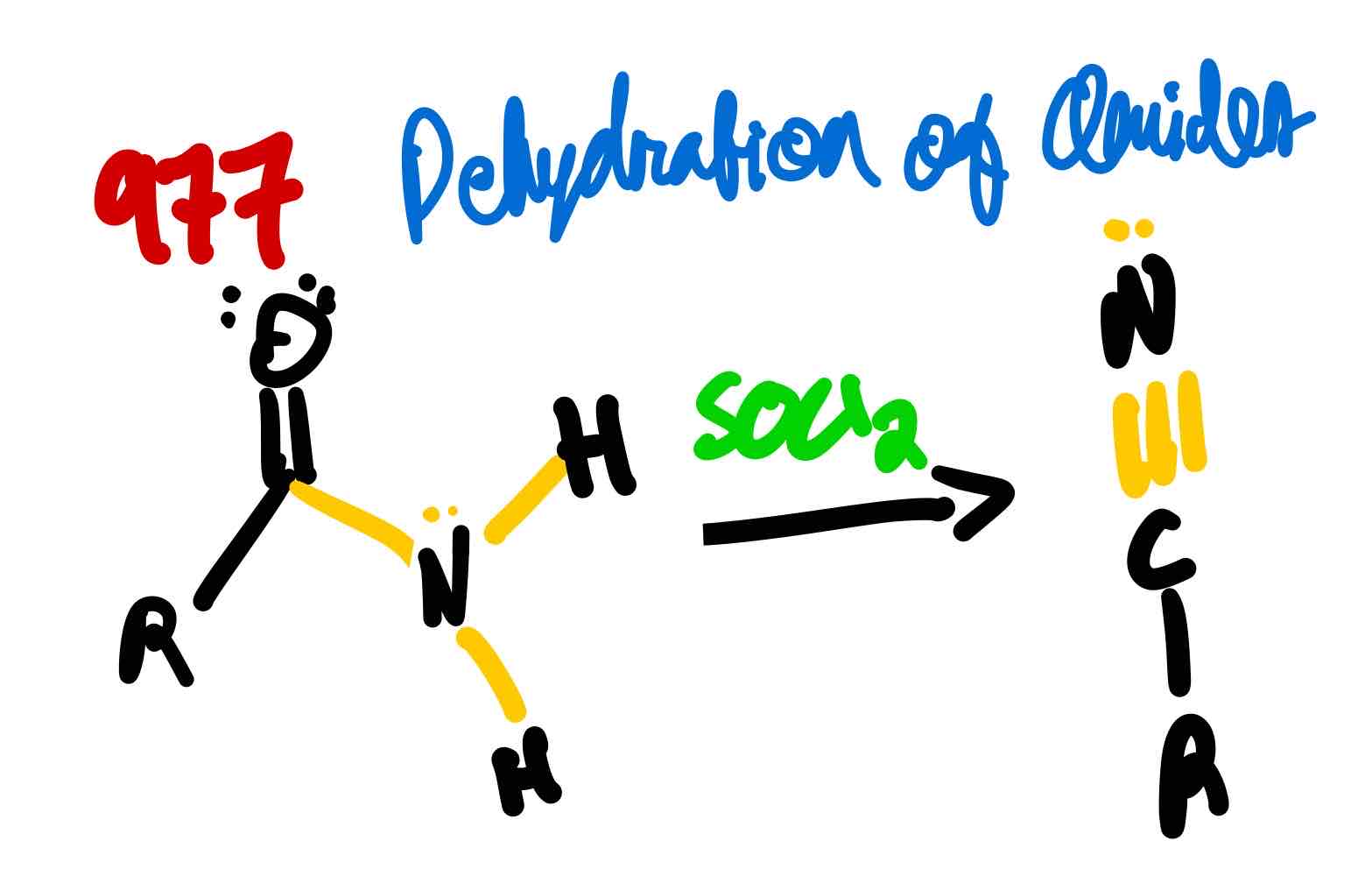
From amide to nitrile, how?
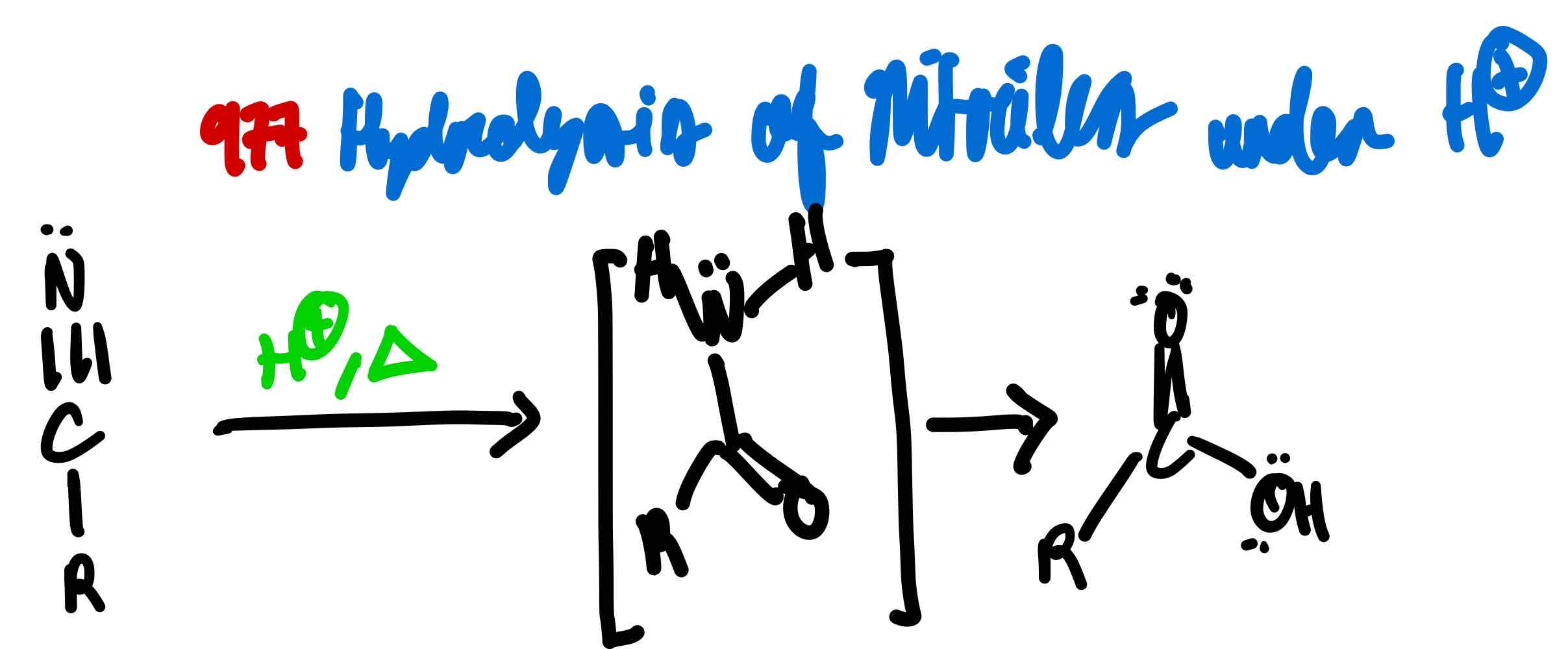
From nitrile to carboxylic acid in acidic conditions, how?
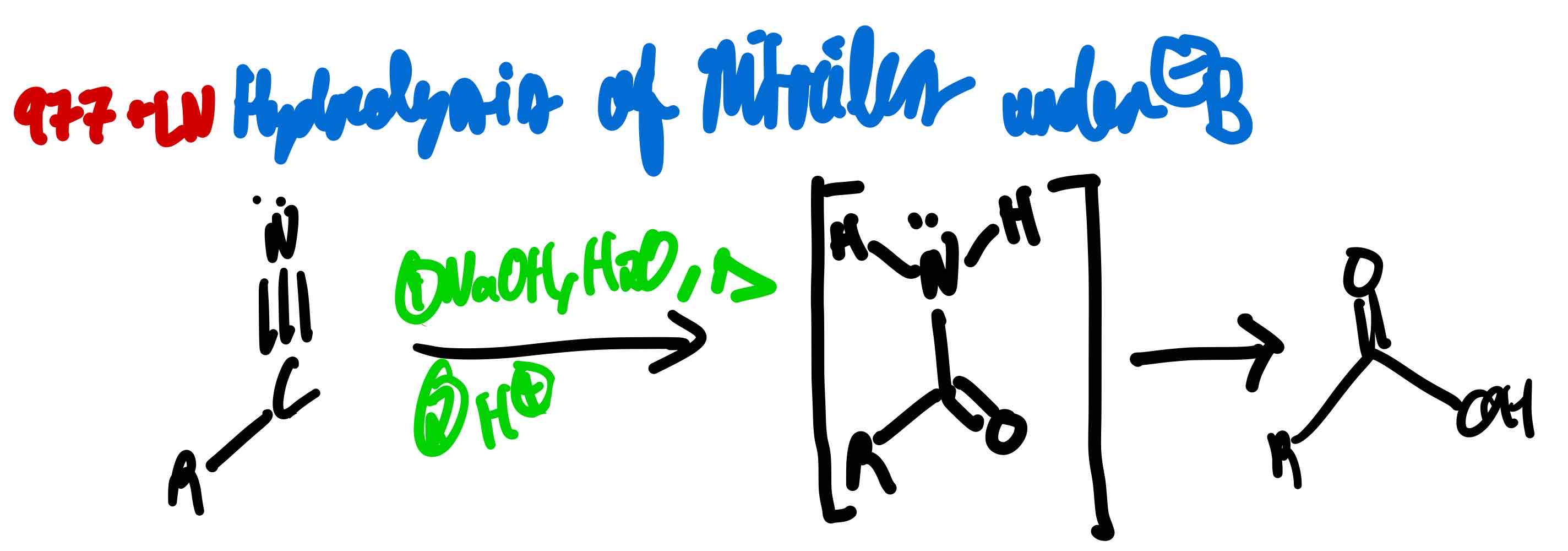
From nitrile to carboxylic acid in basic conditions, how?
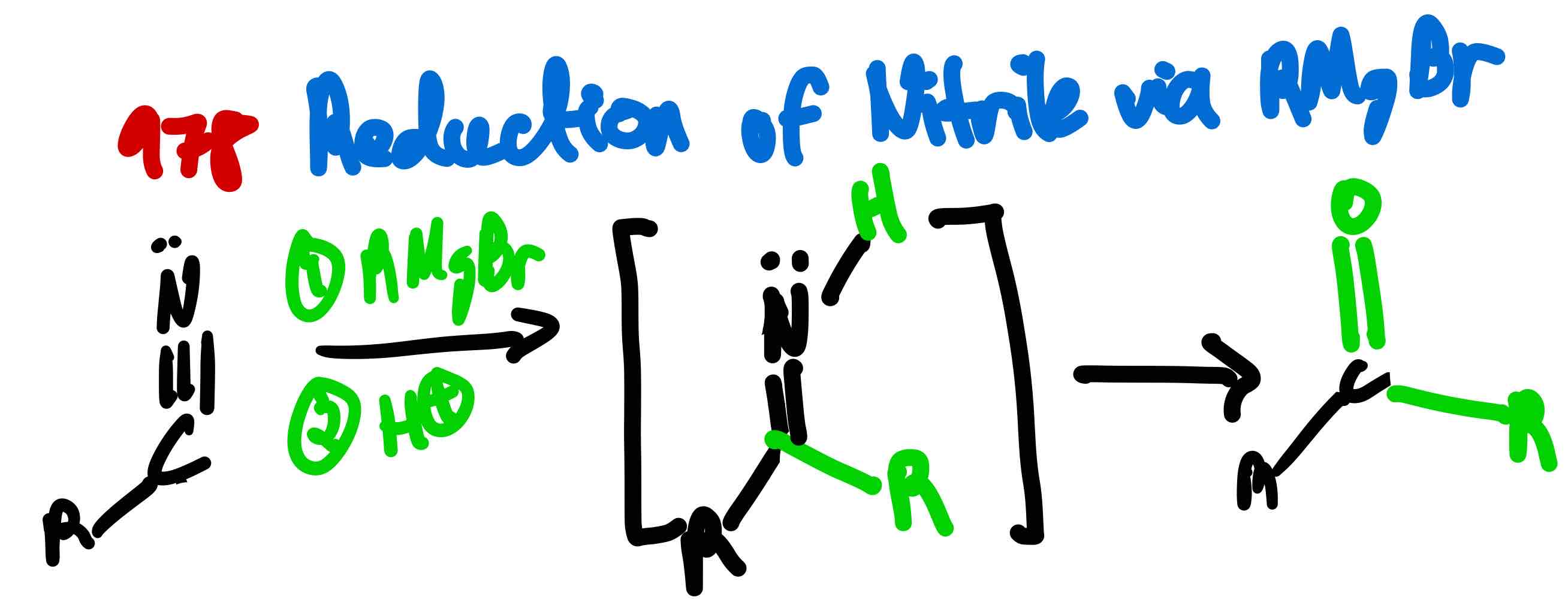
From nitrile to ketone, how?
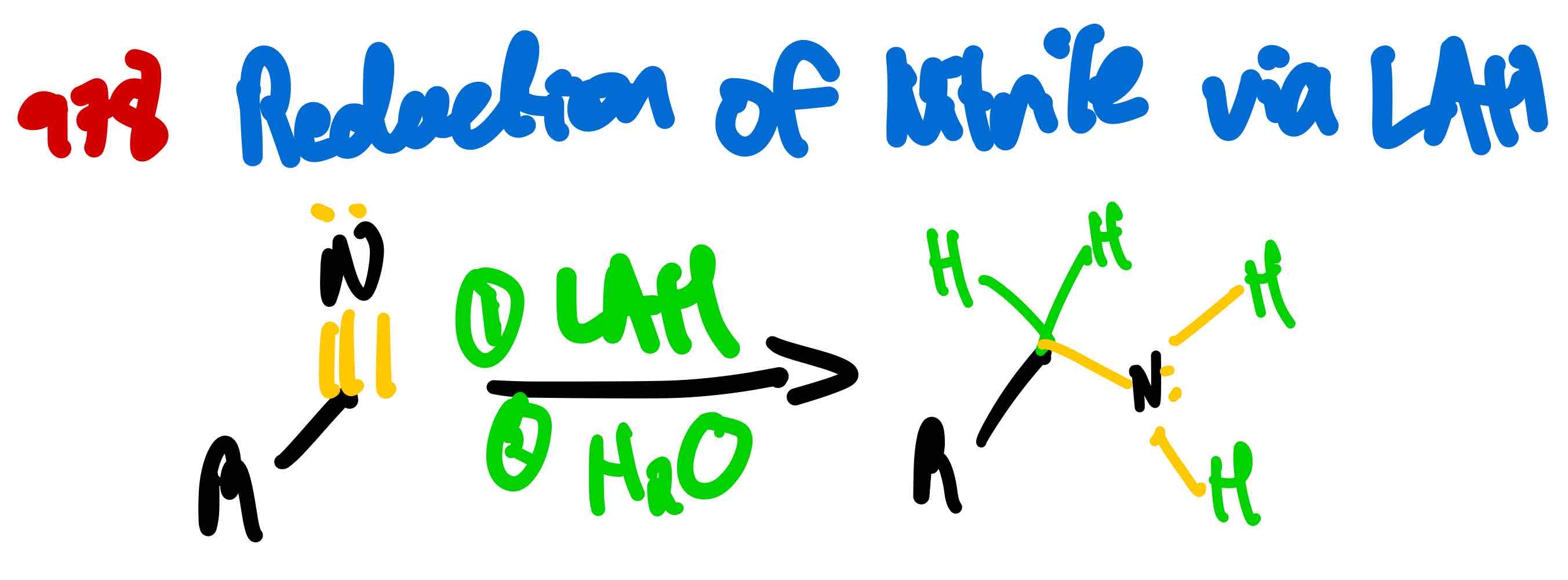
From nitrile to amine, how?