Chem mock
0.0(0)
Card Sorting
1/148
Last updated 7:48 PM on 1/20/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
149 Terms
1
New cards
First ionisation energy
The first ionisation energy is the energy required to remove an electron from every atom in a mole of atomic gas, to produce a mole of unipositive gaseous ions.
2
New cards
Nth ionisation energy equation
Xⁿ⁻¹⁺ (g) → e⁻ + Xⁿ⁺
3
New cards
Ionisation energy ___ down a group
Decreases
4
New cards
Ionisation energy ___ across a period
Increases
5
New cards
Melting point ___ down group 2.
decreases
6
New cards
Why does magnesium have an anomalously low meting point?
it has a different crystal structure to the rest of group 2
7
New cards
Group 2 metals + water →
metal hydroxides + hydrogen
8
New cards
Reactivity ___ as you go down group 2
Increases
9
New cards
Exception to the group 2 reactivity trend
Beryllium
10
New cards
Solubility trend of group 2 hydroxides
Increases down group 2
11
New cards
Solubility trend of group 2 sulphates
decreases down group 2
12
New cards
Group 2 hydroxide that is sparingly soluble
Magnesium hydroxide
13
New cards
Completely insoluble group 2 sulphate
barium sulphate
14
New cards
Reactivity of group 2 metals ___ down the group
Increases
15
New cards
Group 2 metal + oxygen →
metal oxide
16
New cards
Group 2 metals + dilute acids →
metal sulphate + hydrogen gas
17
New cards
Group 2 salts solubility trend
Less soluble as you go down the group
18
New cards
Avogadro’s constant
6.02 x 10²³
19
New cards
Number of moles =
number of particles/Avogadro’s constant
20
New cards
Ideal gas equation =
pV = nRT
21
New cards
What is the value for R in the ideal gas equation?
8.314
22
New cards
What does p represent in the ideal gas equation?
Pressure in Pa
23
New cards
What does V represent in the ideal gas equation?
Volume in m³
24
New cards
What does n represent in the ideal gas equation?
Number of moles
25
New cards
What does T represent in the ideal gas equation?
Temperature in K
26
New cards
How to convert between °C and K?
°C + 273
27
New cards
How to convert between Pa and kPa?
Divide by 1000
28
New cards
How to convert between dm³ and m³?
Divide by 1000
29
New cards
Gas volume equation
n = volume in dm³/24
30
New cards
Equation for finding moles in the ideal gas equation?
n = pV/RT
31
New cards
Equation for finding pressure in the ideal gas equation?
p = nRT/V
32
New cards
Conc, mol, vol equation
c=n/v
33
New cards
Name two indicators used for acid/base titrations
Methyl orange and phenolphthalein
34
New cards
What is a standard solution?
A solution that has a precisely known concentration
35
New cards
Percentage yield =
(Actual yield/theoretical yield) x 100
36
New cards
% atom economy =
Molecular mass of desired product/sum of all molecular masses of products x 100
37
New cards
Reactions with ___ atom economies are less sustainable.
Low
38
New cards
A low atom economy means …
There is a lot of product wasted
39
New cards
Oxidation number of oxygen in a peroxide?
-1
40
New cards
Oxidation number of hydrogen in a metal hydride?
-1
41
New cards
Total electron configuration list
1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s²4d¹⁰
42
New cards
A covalent bond is …
The strong electrostatic attraction between a shared pair of electrons and the nuclei of the bonded atoms.
43
New cards
Linear shape bond angle
180
44
New cards
What proportions are needed for linear shape?
two electron pairs
45
New cards
Trigonal planar bond angle
120
46
New cards
What proportions are needed for trigonal planar shape?
three bonding pairs
47
New cards
Tetrahedral bond angle
109\.5
48
New cards
What proportions are needed for tetrahedral shape?
4 bonding pairs
49
New cards
Trigonal pyramidal bond angle
107
50
New cards
What proportions are needed for trigonal pyramidal shape?
3 bonding pairs and 2 lone pairs
51
New cards
Non-linear bond angle
120
52
New cards
What proportions are need for non-linear shape?
2 bonding pairs and 2 lone pairs
53
New cards
Trigonal bipyramidal bond angle
3 = 120, 2 = 90
54
New cards
What proportions are needed for trigonal bipyramidal shape?
5 bonding pairs
55
New cards
Octahedral bond angle
90
56
New cards
What proportions are needed for octahedral shape?
6 bonding pairs
57
New cards
A higher number on the Pauling Scale means…
more electronegative
58
New cards
Electronegativity increases towards which element?
fluorine
59
New cards
Polar molecules have an overall ___
dipole
60
New cards
Difference in electronegativity values to be ionic
>2.0
61
New cards
Difference in electronegativity value to be polar
between 0.4 and 2.0
62
New cards
Difference in electronegativity values to be non-polar covalent.
63
New cards
What is a dipole?
A difference in charge between the two atoms caused by a shift in electron density in the bond.
64
New cards
Hydrogen bonds only happen when hydrogen is covalently bonded to ____
fluorine, nitrogen, and oxygen
65
New cards
Silver nitrate is used to test for ____?
Halide ions
66
New cards
Chloride ion colour in silver nitrate
White
67
New cards
Bromide ion colour in silver nitrate
Cream
68
New cards
Iodide ion colour in silver nitrate ?
Yellow
69
New cards
Which halide will dissolve in dilute ammonia?
Chloride ions
70
New cards
Which halide only dissolves in conc. ammonia?
Bromide ions
71
New cards
Which halide persists in concentrated ammonia?
Iodine
72
New cards
Order of ion tests
Test for carbonates → test for sulphates → test for halides
73
New cards
What reagent tests for sulphate ions?
Barium nitrate
74
New cards
Damp red litmus paper is used to test for ….. ?
Ammonium ions
75
New cards
Standard enthalpy change of combustion
Standard enthalpy change when of combustion when one mole of a compound is completely burned in oxygen under its standard conditions at RTP.
76
New cards
Enthalpy change of neutralisation
The enthalpy change when an acid and alkali react together to produce one mole of water.
77
New cards
Average mole enthalpy
Average bond enthalpy is the energy needed to break one mole of bonds in the gas phase, averaged over many different compounds,
78
New cards
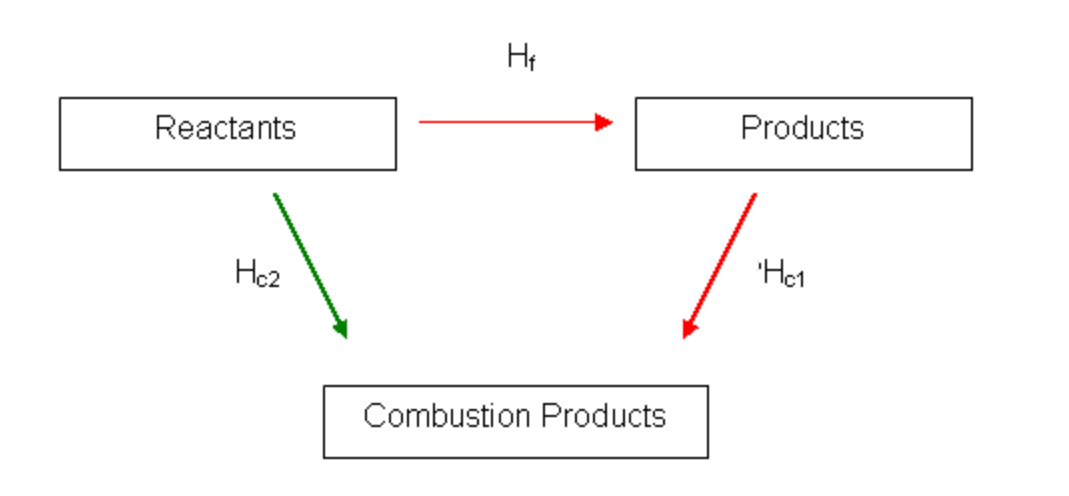
Hess cycle of combustion
79
New cards
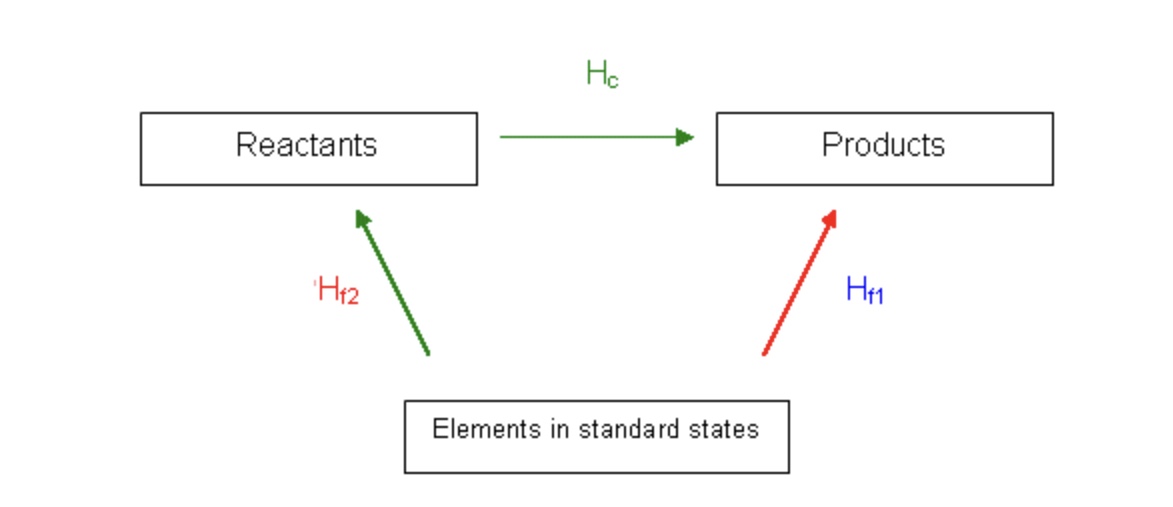
Hess cycle of formation
80
New cards
Way to remember arrow direction for hess cycle of combustion
Hot people go down
81
New cards
Standard conditions
298K and 101kPa
82
New cards
Catalyst for hydration of alkene into an alcohol
Acid catalyst (H₃PO₃/H₂SO₄)
\
\
83
New cards
Electrophilic addition catalyst
Ni
84
New cards
Why are are amines bases?
Because the one pair on the nitrogen atom forms a dative covalent bond and accepts the proton ( H+)
85
New cards
Bases are x acceptors and x donors
Proton, electron
86
New cards
What catalyst do you need for polyester hydrolysis?
Base
87
New cards
What catalyst do you need for polyamide hydrolysis?
Acid
88
New cards
Produce an aliphatic amine from a haloalkane
Haloalkane, ammonia (dissolved in ethanol)
89
New cards
Produce an aromatic amine by reducing an aromatic nitro compound
* make aromatic salt by heating nitro compound, tin metal, and conc. HCl under reflux
* Turn ammonium salt into an an aromatic amine by adding an alkali like NaOH
* Turn ammonium salt into an an aromatic amine by adding an alkali like NaOH
90
New cards
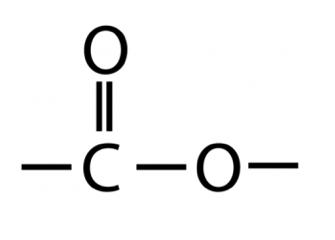
Functional group of an ester
91
New cards
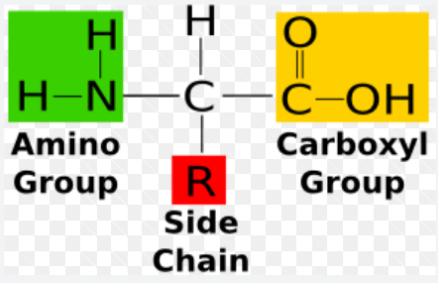
Amino acid basic structure
92
New cards
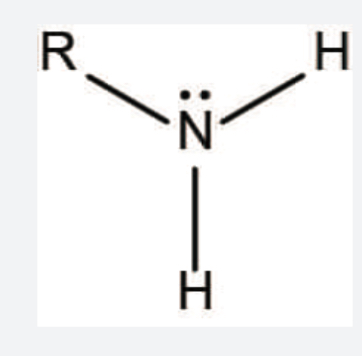
Amine functional group
93
New cards
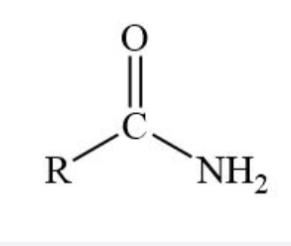
Amide functional group
94
New cards
Alkane to haloalkane reagents and conditions
Halogen and UV
95
New cards
Alkene to alkane reagents and conditions
H₂ and Ni
96
New cards
Alkene to haloalkane reagents and conditions
Hydrogen halide
97
New cards
Alkane to haloalkane reaction
Radical substitution
98
New cards
Alkene to alkane reaction
Hydrogenation
99
New cards
Alkene to haloalkane reaction
Halogenation
100
New cards
Alkene to alcohol reagents and conditions
Steam and H₃PO₄