AQA GCSE Chemistry Paper 2
1/301
Earn XP
Description and Tags
This set should (hopefully) contain everything you need to know for AQA GCSE Chemistry Paper 2. Made using the specification and Freesciencelessons on YouTube. This is designed to be used in Term → Definition mode. You can do it the other way around for extra revision, but it will not be framed as a question. Topic 6 - The rate and extent of chemical change: Cards 1-32 Topic 7 - Organic chemistry: Cards 33-114 Topic 8 - Chemical analysis: Cards 115-159 Topic 9 - Chemistry of the atmosphere: Cards 160-193 Topic 10 - Using resources: Cards 194-302 (Topics 1-5 are in the Paper 1 flashcards) (Note: You may need to apply concepts from Topics 1-3 in Paper 2.) Cards starting with ᵀ are triple/separate/chemistry only. Cards starting with ᴴ are Higher tier only. (Cards starting with ᵀᴴ are both.) If you want to remove these cards, star all the other cards and then study with starred cards only. Note: Chromatography is covered in Topics 1 and 8. It is included in the Paper 2 flashcards since there is a required practical about chromatography in Topic 8. Note: If you are doing triple science, you need to be able to write balanced symbol equations for the reactions to produce the six insoluble metal hydroxides that you need to know. However, depending on which spectator ion is used, the numbers in the equation may change. To account for this, I wrote the ionic equations in those flashcards. However, ionic equations are Higher tier only. So, if you’re doing Foundation tier triple science, you need to be able to write balanced symbol equations for those reactions without knowing the ionic equations (which is arguably harder).
Chemistry
Equilibrium
GCSE Chemistry
AQA
aqa
chemistry
gcse
higher
foundation
triple
combined
chemistry only
paper 2
the rate and extent of chemical change
organic chemistry
chemical analysis
chemistry of the atmosphere
using resources
science
trilogy
topic 6
topic 7
topic 8
topic 9
topic 10
HT
rate of reaction
calculating rates of reactions
factors which affect the rates of chemical reactions
collision theory and activation energy
catalysts
reversible reactions and dynamic equilibrium
reversible reactions
energy changes and reversible reactions
equilibrium
the effect of changing conditions on equilibrium
the effect of changing concentration
the effect of temperature changes on equilibrium
the effect of pressure changes on equilibrium
carbon compounds as fuels and feedstock
crude oil, hydrocarbons and alkanes
fractional distillation and petrochemicals
properties of hydrocarbons
cracking and alkenes
reactions of alkenes and alcohols
structure and formulae of alkenes
reactions of alkenes
alcohols
carboxylic acids
synthetic and naturally occurring polymers
addition polymerisation
condensation polymerisation
amino acids
DNA (deoxyribonucleic acid) and other naturally occurring polymers
purity, formulations and chromatography
pure substances
formulations
chromatography
identification of common gases
test for hydrogen
test for oxygen
test for carbon dioxide
test for chlorine
identification of ions by chemical and spectroscopic means
flame tests
metal hydroxides
carbonates
halides
sulfates
instrumental methods
flame emission spectroscopy
the composition and evolution of the earth’s atmosphere
the proportions of different gases in the atmosphere
the earth’s early atmosphere
how oxygen increased
how carbon dioxide decreased
carbon dioxide and methane as greenhouse gases
greenhouse gases
human activities which contribute to an increase in greenhouse gases in the atmosphere
global climate change
the carbon footprint and its reduction
common atmospheric pollutants and their sources
atmospheric pollutants from fuels
properties and effects of atmospheric pollutants
using the earth’s resources and obtaining potable water
using the earth’s resources and sustainable development
potable water
waste water treatment
alternative methods of extracting metals
life cycle assessment and recycling
life cycle assessment
ways of reducing the use of resources
using materials
corrosion and its prevention
alloys as useful materials
ceramics, polymers and composites
the Haber process and the use of NPK fertilisers
the Haber process
production and use of NPK fertilisers
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
302 Terms
How can the rate of a chemical reaction be found?
By measuring the quantity of a reactant used or the quantity of product formed over time
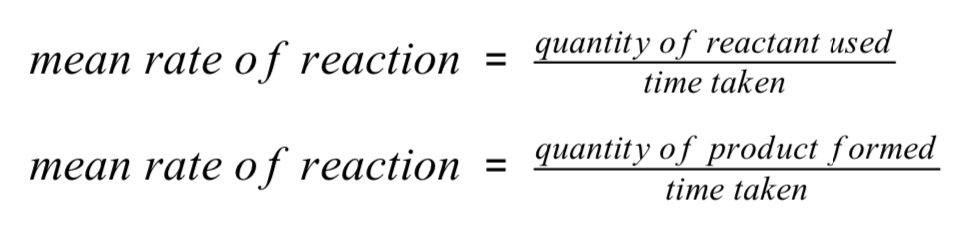
How can the quantity of a reactant or product be measured?
By the mass in grams or by a volume in cm³
(Higher Tier only) Or in terms of moles
How may the units of rate of reaction be given?
g/s or cm³/s
(Higher Tier only) or mol/s
What factors affect the rates of chemical reactions?
The concentrations of reactants in solution, the pressure of reacting gases, the surface area of solid reactants, the temperature and the presence of catalysts
How does increasing the concentrations of reactants in solution affect the rate of chemical reactions?
It increases the frequency of collisions and so increases the rate of reaction
How does increasing the pressure of reacting gases affect the rate of chemical reactions?
It increases the frequency of collisions and so increases the rate of reaction
How does increasing the surface area of solid reactants affect the rate of chemical reactions?
It increases the frequency of collisions and so increases the rate of reaction
How does increasing the temperature affect the rates of chemical reactions?
It increases the frequency of collisions and makes the collisions more energetic, and so increases the rate of reaction
Describe how to investigate how changes in concentration affect the rate of reaction by a method involving measuring the volume of a gas produced.
Use a measuring cylinder to place 50cm³ of hydrochloric acid into a conical flask.
Attach the conical flask to a bung and delivery tube. Place the delivery tube into a container filled with water.
Place an upturned measuring cylinder also filled with water into the delivery tube.
Add a 3cm strip of magnesium to the hydrochloric acid and start a stopwatch.
Every ten seconds, measure the volume of hydrogen gas in the measuring cylinder. Continue until no more hydrogen is given off.
Repeat the experiment using different concentrations of hydrochloric acid.
Describe how to investigate how changes in concentration affect the rate of reaction by a method involving a change in colour or turbidity.
- Use a measuring cylinder to put 10cm³ of sodium thiosulfate solution into a conical flask.
- Place the conical flask onto a printed black cross.
- Add 10cm³ of hydrochloric acid into the conical flask.
- Swirl the solution and start a stopwatch.
- Look down through the top of the flask. After a certain time the solution will turn cloudy. Stop the clock when you can no longer see the cross.
- Carry out the experiment again using lower concentrations of sodium thiosulfate solution.
- Repeat the whole experiment and calculate mean values for each concentration of sodium thiosulfate solution.
What is collision theory?
Collision theory explains how various factors affect rates of reactions. According to this theory, chemical reactions can occur only when reacting particles collide with each other and with sufficient energy.
What is activation energy?
The minimum amount of energy that particles must have to react
How would changing the sizes of pieces of a reacting solid affect the rate of a reaction?
Smaller sized pieces of solid reactant have a greater surface area to volume ratio than larger pieces. This means they have more particles on the surface so there are more collisions per second. This increases the rate of reaction.
What are catalysts?
Catalysts change the rate of chemical reactions but are not used up during the reaction
Do different reactions need the same catalysts, or different catalysts?
Different catalysts
What act as catalysts in biological systems?
Enzymes
Explain catalytic action in terms of activation energy.
Catalysts increase the rate of reaction by providing a different pathway for the reaction that has a lower activation energy.
Draw a reaction profile for a catalysed and an uncatalysed exothermic reaction.
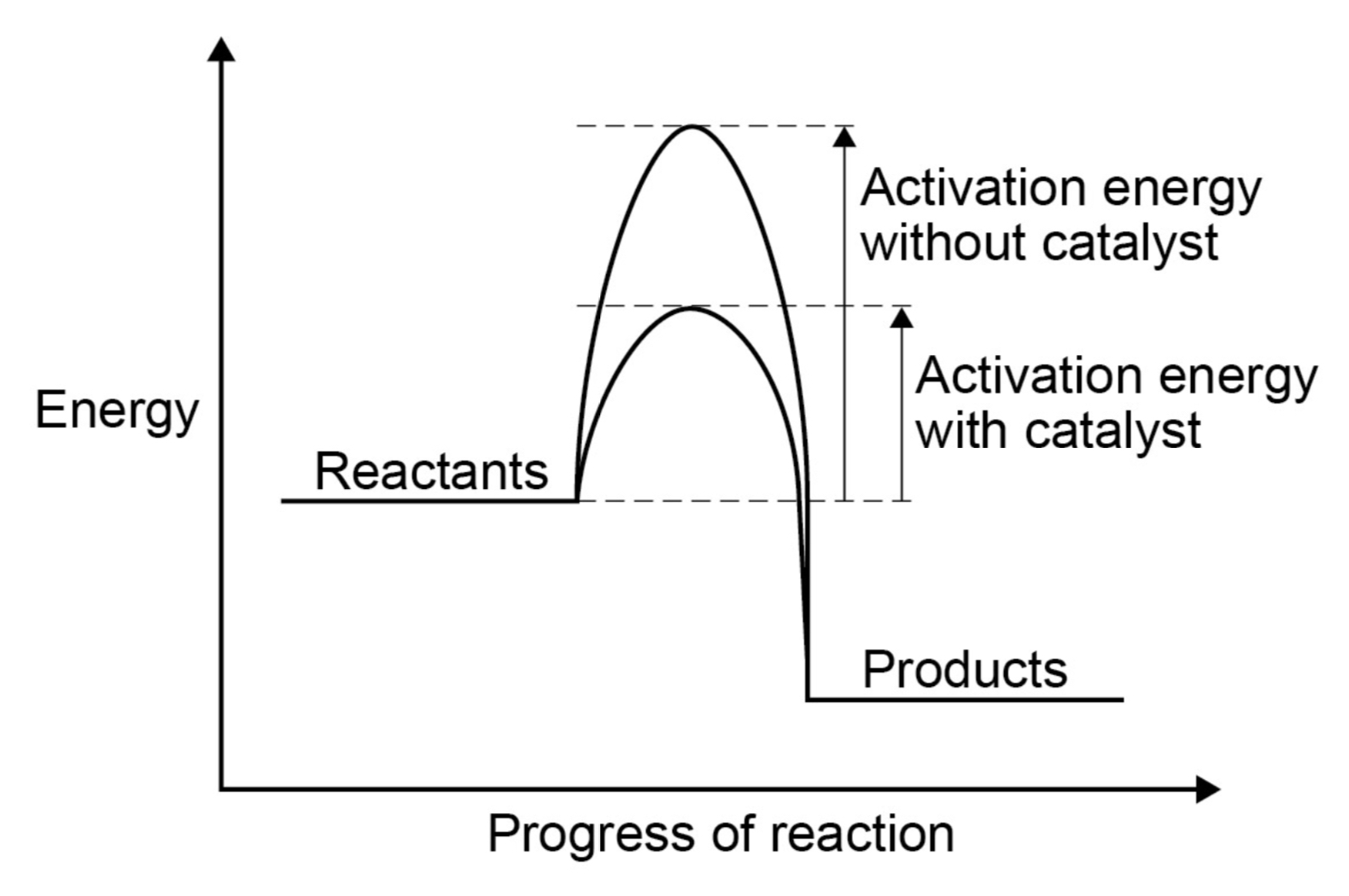
How can catalysts be identified in reactions?
From their effect on the rate of reaction, and because they are not included in the chemical equation for the reaction
What are reversible reactions?
Reactions where the products of the reaction can react to produce the original reactants
How are reversible reactions represented?
A + B ⇌ C + D
Give an example of a reversible reaction whose direction can be changed by changing the conditions.
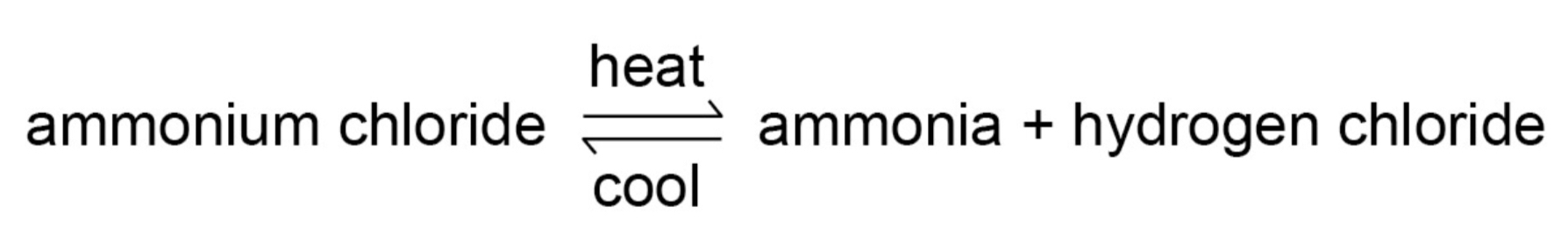
What is true about the energy changes in reversible reactions?
If a reversible reaction is exothermic in one direction, it is endothermic in the opposite direction. The same amount of energy is transferred in each case.
Give an example of a reversible reaction that is endothermic in one direction and exothermic in the other.
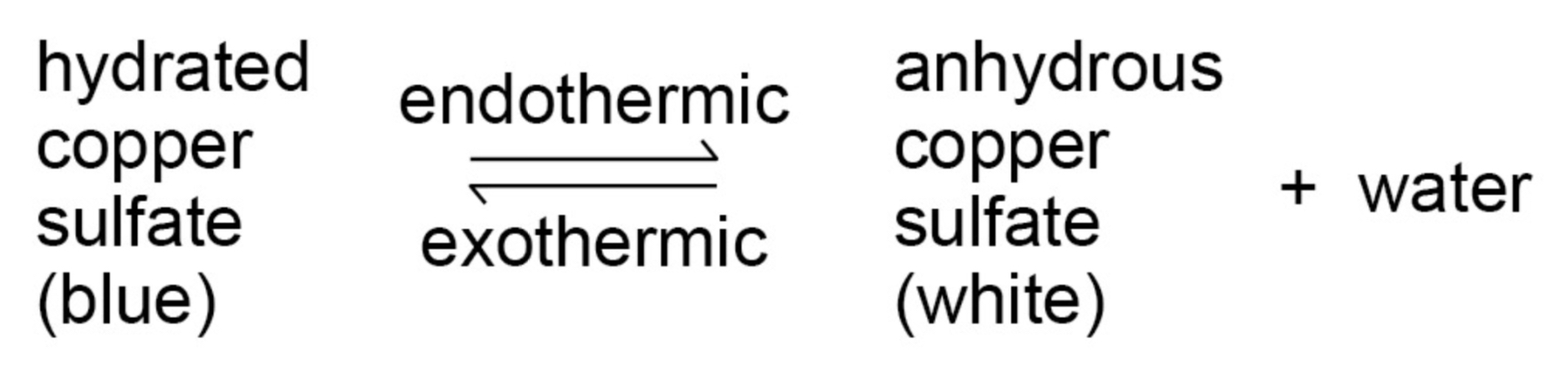
What is equilibrium (regarding reversible reactions)?
When a reversible reaction occurs in apparatus which prevents the escape of reactants and products, equilibrium is reached when the forward and reverse reactions occur at exactly the same rate.
ᴴ What do the relative amounts of all the reactants and products of a reaction at equilibrium depend on?
The conditions of the reaction
ᴴ What happens if a system is at equilibrium and a change is made to any of the conditions?
The system responds to counteract the change
ᴴ How can the effects of changing conditions on a system at equilibrium be predicted?
Using Le Chatelier’s Principle
ᴴ What happens if the concentration of one of the reactants or products in a system at equilibrium is changed?
The system is no longer at equilibrium and the concentrations of all the substances will change until equilibrium is reached again.
If the concentration of a reactant is increased, more products will be formed until equilibrium is reached again.
If the concentration of a product is decreased, more reactants will react until equilibrium is reached again.
ᴴ What happens if the temperature of a system at equilibrium is increased?
The relative amount of products at equilibrium increases for an endothermic reaction. The relative amount of products at equilibrium decreases for an exothermic reaction.
ᴴ What happens if the temperature of a system at equilibrium is decreased?
The relative amount of products at equilibrium decreases for an endothermic reaction. The relative amount of products at equilibrium increases for an exothermic reaction.
ᴴ What happens if the pressure of a gaseous reaction at equilibrium is changed?
An increase in pressure causes the equilibrium position to shift towards the side with the smaller number of molecules as shown by the symbol equation for that reaction. A decrease in pressure causes the equilibrium position to shift towards the side with the larger number of molecules as shown by the symbol equation for that reaction.
What is crude oil?
Crude oil is a finite resource found in rocks. Crude oil is the remains of an ancient biomass consisting mainly of plankton that was buried in mud.
What does crude oil contain?
Crude oil is a mixture of a very large number of compounds. Most of the compounds in crude oil are hydrocarbons, which are molecules made up of hydrogen and carbon atoms only.
What are most of the hydrocarbons in crude oil?
Alkanes
What is the general formula for the homologous series of alkanes?
CₙH₂ₙ₊₂
What are the first four members of the alkanes?
Methane, ethane, propane and butane
How can alkane molecules be represented?
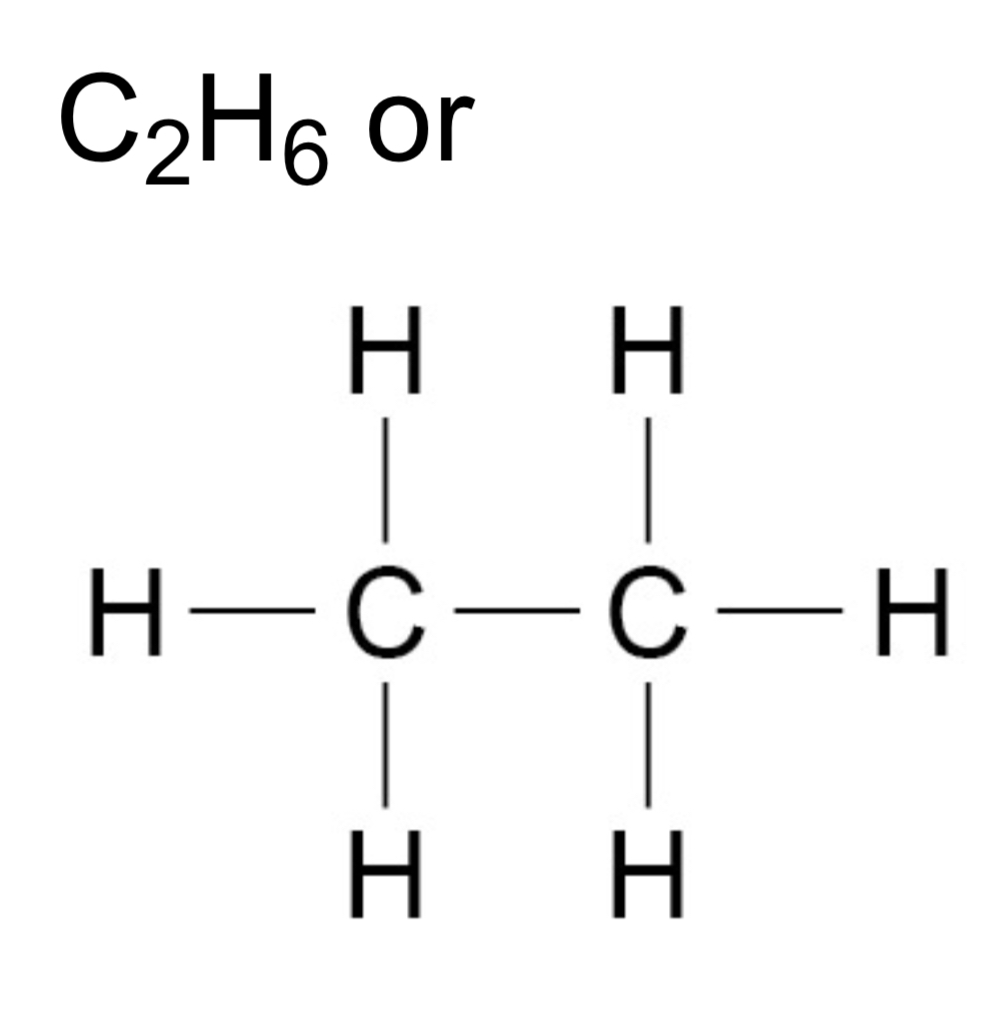
What can the many hydrocarbons in crude oil be separated into?
Fractions, each of which contains molecules with a similar number of carbon atoms
What can fractions that crude oil was separated into be processed to produce?
Fuels and feedstock for the petrochemical industry
What fuels which we depend on for our modern lifestyle are produced from crude oil?
Petrol, diesel oil, kerosene, heavy fuel oil and liquefied petroleum gases
What useful materials on which modern life depends are produced by the petrochemical industry?
Solvents, lubricants, polymers and detergents
Why do the vast array of natural and synthetic carbon compounds occur?
Because of the ability of carbon atoms to form families of similar compounds
How does the fractional distillation of crude oil work?
The crude oil is heated to a very high temperature. This causes the crude oil to boil. All of the hydrocarbons evaporate and turn into a gas. The crude oil vapour is fed into the fractional distillation column. The column is hotter at the bottom and cooler at the top. The hydrocarbon vapours rise up the column. Hydrocarbons condense when they reach their boiling point. The liquid fractions are then removed. The remaining hydrocarbons continue moving up the column. These condense when they reach their boiling points. Very long chain hydrocarbons have very high boiling points. These hydrocarbons are removed from the bottom of the column. Very short chain hydrocarbons have very low boiling points. These do not condense. They are removed from the top of the column as gases.
What properties of hydrocarbons depend on the size of their molecules?
Boiling point, viscosity and flammability
What influences how hydrocarbons are used as fuels?
Their properties (which depend on their size)
How do the boiling points of hydrocarbons change with increasing molecular size?
They increase
How does the viscosity of hydrocarbons change with increasing molecular size?
It increases
How does the flammability of hydrocarbons change with increasing molecular size?
It decreases
What does the combustion of hydrocarbon fuels release?
Energy
What is oxidised during the combustion of a hydrocarbon?
The carbon and hydrogen in the fuel
What does the complete combustion of a hydrocarbon produce?
Carbon dioxide and water
What can hydrocarbons be broken down (cracked) to produce?
Smaller, more useful molecules: alkanes and alkenes
What are two methods of cracking?
Catalytic cracking and steam cracking
What are the conditions used for catalytic cracking?
High temperature and a catalyst
What are the conditions used for steam cracking?
High temperature and steam
Which are more reactive: alkanes or alkenes?
Alkenes
What is the test for alkenes?
Alkenes react with bromine water, which is orange, and the bromine water turns colourless
Why are some of the products of cracking useful as fuels?
There is a high demand for fuels with small molecules
What are alkenes used for?
Producing polymers and as starting materials for the production of many other chemicals
ᵀ What are alkenes?
Hydrocarbons with a double carbon-carbon bond
ᵀ What is the general formula for the homologous series of alkenes?
CₙH₂ₙ
ᵀ Why are alkene molecules unsaturated?
Because they contain two fewer hydrogen atoms than the alkane with the same number of carbon atoms
ᵀ What are the first four members of the homologous series of alkenes?
Ethene, propene, butene and pentene
ᵀ How can alkene molecules be represented?
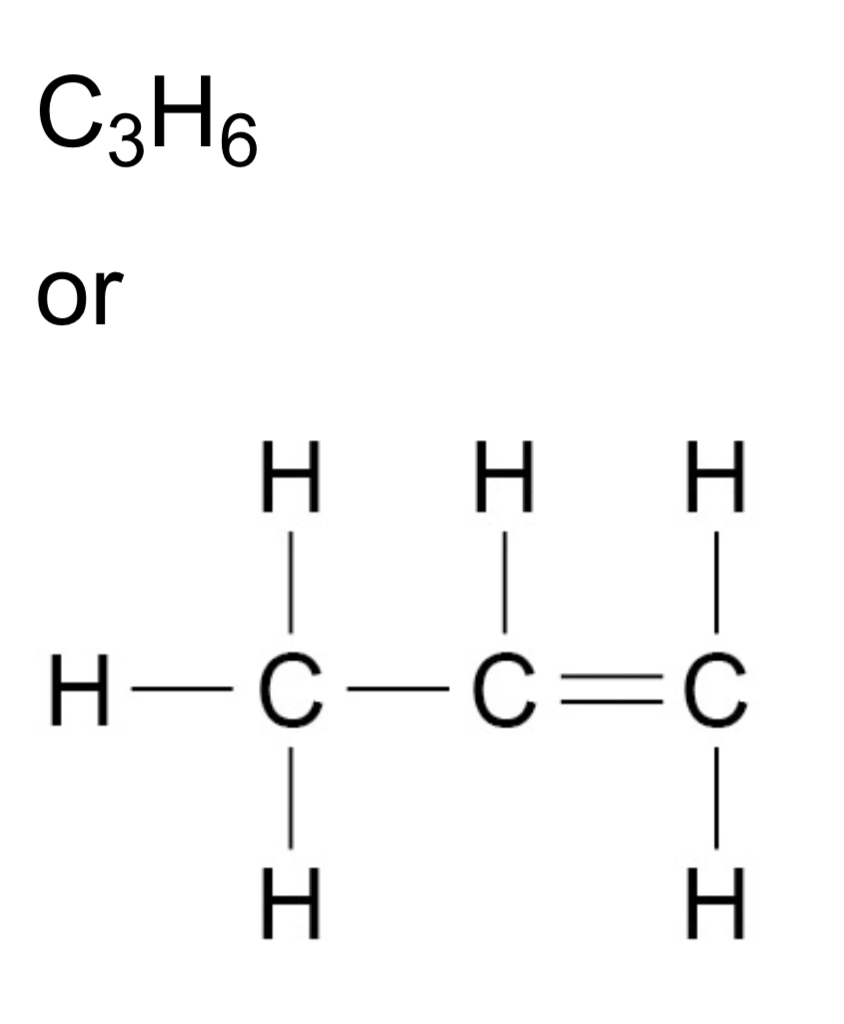
ᵀ What is the functional group of alkenes?
C=C
ᵀ What determines the reactions of organic compounds?
The generality of reactions of functional groups
ᵀ How do alkenes react with oxygen in combustion reactions?
In the same way as other hydrocarbons, but they tend to burn in air with smoky flames because of incomplete combustion
ᵀ Describe the reactions of alkenes with hydrogen.
The two hydrogen atoms are added across the carbon-carbon double bond so that the double bond becomes a single carbon-carbon bond. An alkane is produced.
ᵀ What conditions are needed for alkenes to react with hydrogen?
A temperature of around 150°C and the presence of a nickel catalyst
ᵀ Describe the reactions of alkenes with water.
The water is added across the carbon-carbon double bond so that the double bond becomes a single carbon-carbon bond. An alcohol is produced.
ᵀ What conditions are needed for alkenes to react with water?
The water must be in the form of steam. The temperature must be around 300°C and the pressure around 70 atmospheres. Phosphoric acid is used as a catalyst.
ᵀ Describe the reactions of alkenes with halogens.
The two halogen atoms are added across the carbon-carbon double bond so that the double bond becomes a single carbon-carbon bond. A compound of the form di(halogen)o(alkane) is produced (e.g. dichloroethane, dibromopropane, diiodobutane).
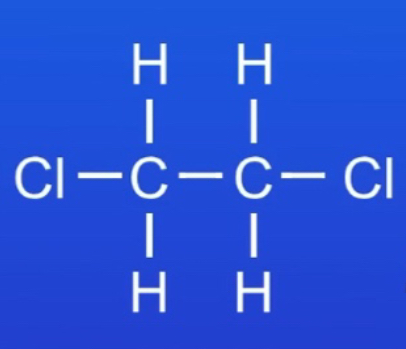
ᵀ Draw the structural formula of the product of the reaction between ethene and chlorine.
(Note: You need to be able to draw the structural formulae for the products of the addition reactions of the first four members of the alkenes and chlorine, bromine and iodine.)
ᵀ What conditions are needed for alkenes to react with halogens?
There are no special conditions
ᵀ What is the functional group of alcohols?
–OH
ᵀ What are the first four members of a homologous series of alcohols?
Methanol, ethanol, propanol and butanol
ᵀ How can alcohols be represented?
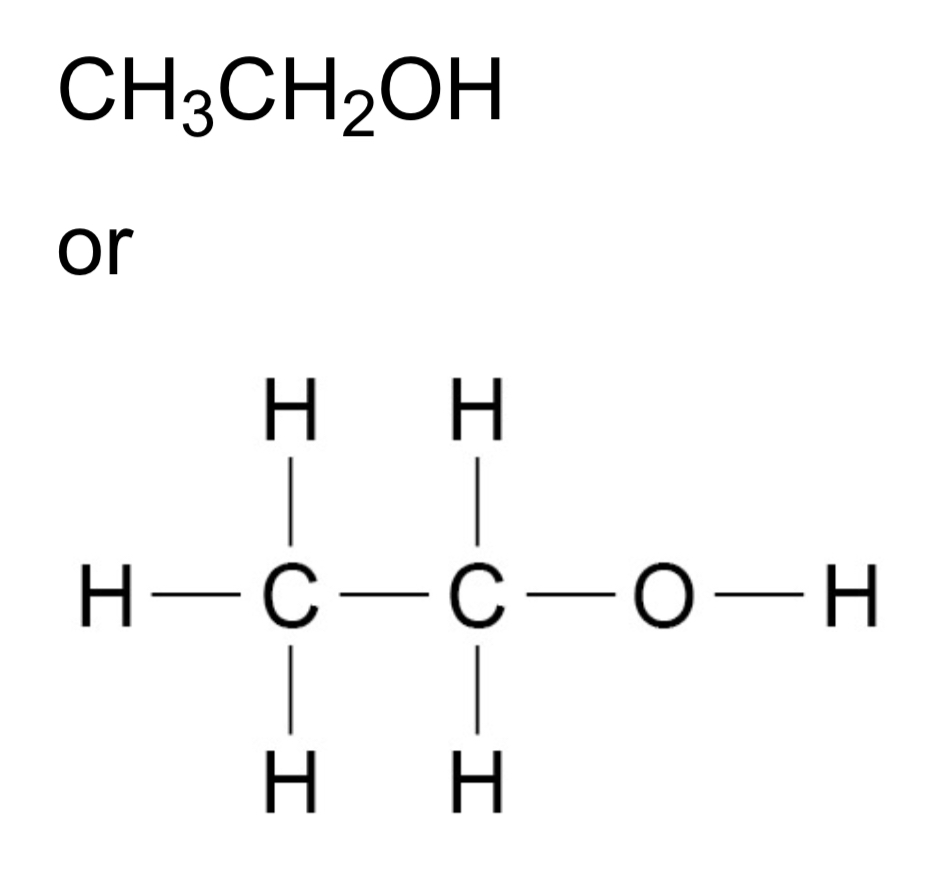
ᵀ What happens when the first four alcohols react with sodium?
Sodium methoxide/ethoxide/propoxide/butoxide and hydrogen gas are produced
ᵀ What happens when the first four alcohols burn in air?
Carbon dioxide and water are produced
ᵀ What happens when the first four alcohols are added to water?
Alcohols are soluble in water and produce neutral solutions. As the number of carbon atoms increases, the solubility decreases.
ᵀ What happens when the first four alcohols react with an oxidising agent?
A carboxylic acid and water are produced
ᵀ What are the main uses of the first four alcohols?
Fuels, solvents, alcoholic drinks
ᵀ When are aqueous solutions of ethanol produced?
When sugar solutions are fermented using yeast
ᵀ What are the conditions used for fermentation of sugar using yeast?
The temperature should be around 30°C. The reaction must take place in anaerobic conditions.
ᵀ What is the functional group of carboxylic acids?
–COOH
ᵀ What are the first four members of a homologous series of carboxylic acids?
Methanoic acid, ethanoic acid, propanoic acid and butanoic acid
ᵀ How can the structures of carboxylic acids be represented?
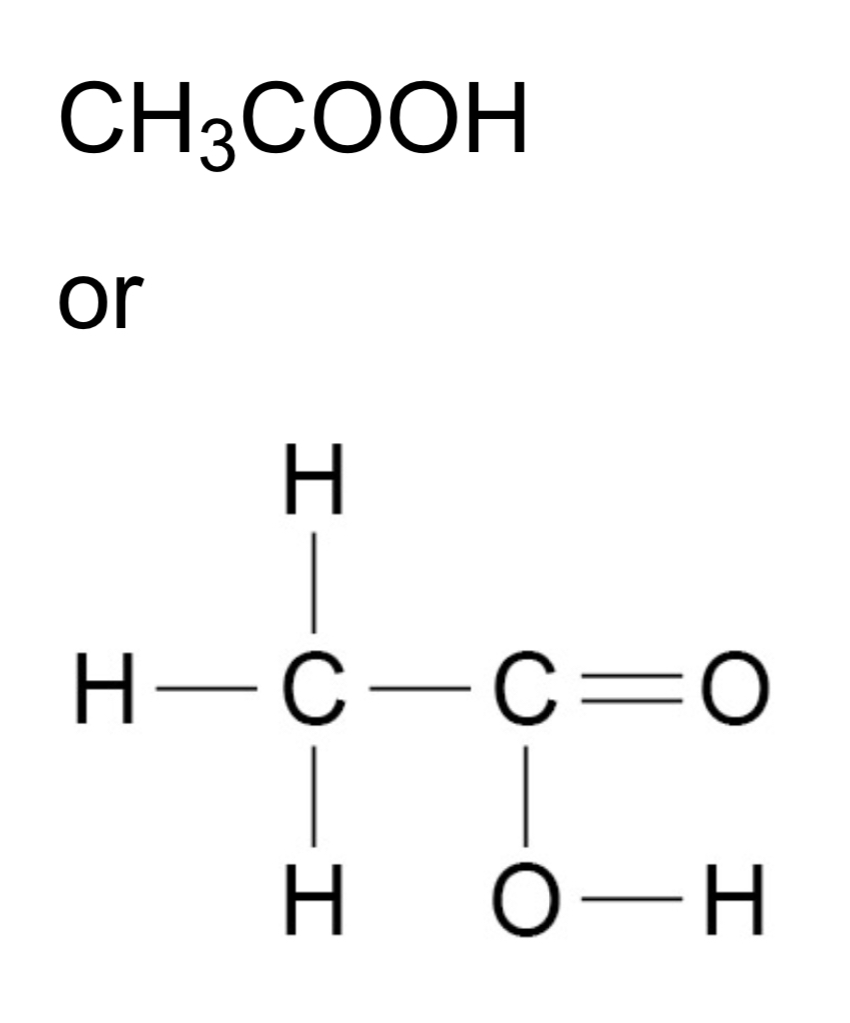
ᵀ What happens when the first four carboxylic acids react with carbonates?
A salt (e.g. sodium ethanoate), carbon dioxide and water are produced
ᵀ What happens when the first four carboxylic acids dissolve in water?
They ionise to produce an ion (e.g. ethanoate) and H⁺
ᵀ What happens when the first four carboxylic acids react with alcohols?
An ester and water are produced
ᵀᴴ Why are carboxylic acids weak acids?
In water, carboxylic acids ionise to produce an ion (e.g. ethanoate) and H⁺. This is a reversible reaction, so the ethanoate ion and H⁺ can recombine to form the carboxylic acid. Because carboxylic acids only partially ionise in aqueous solutions, they are weak acids. Carboxylic acids have a higher pH than strong acids.
ᵀ What ester is produced when ethanoic acid and ethanol react?
Ethyl ethanoate
ᵀ In what type of reaction can alkenes be used to make polymers such as poly(ethene) and poly(propene)?
Addition polymerisation
ᵀ What happens in addition polymerisation reactions?
Many small molecules (monomers) join together to form very large molecules (polymers)
ᵀ Draw a diagram to represent the formation of poly(ethene) from ethene monomers.
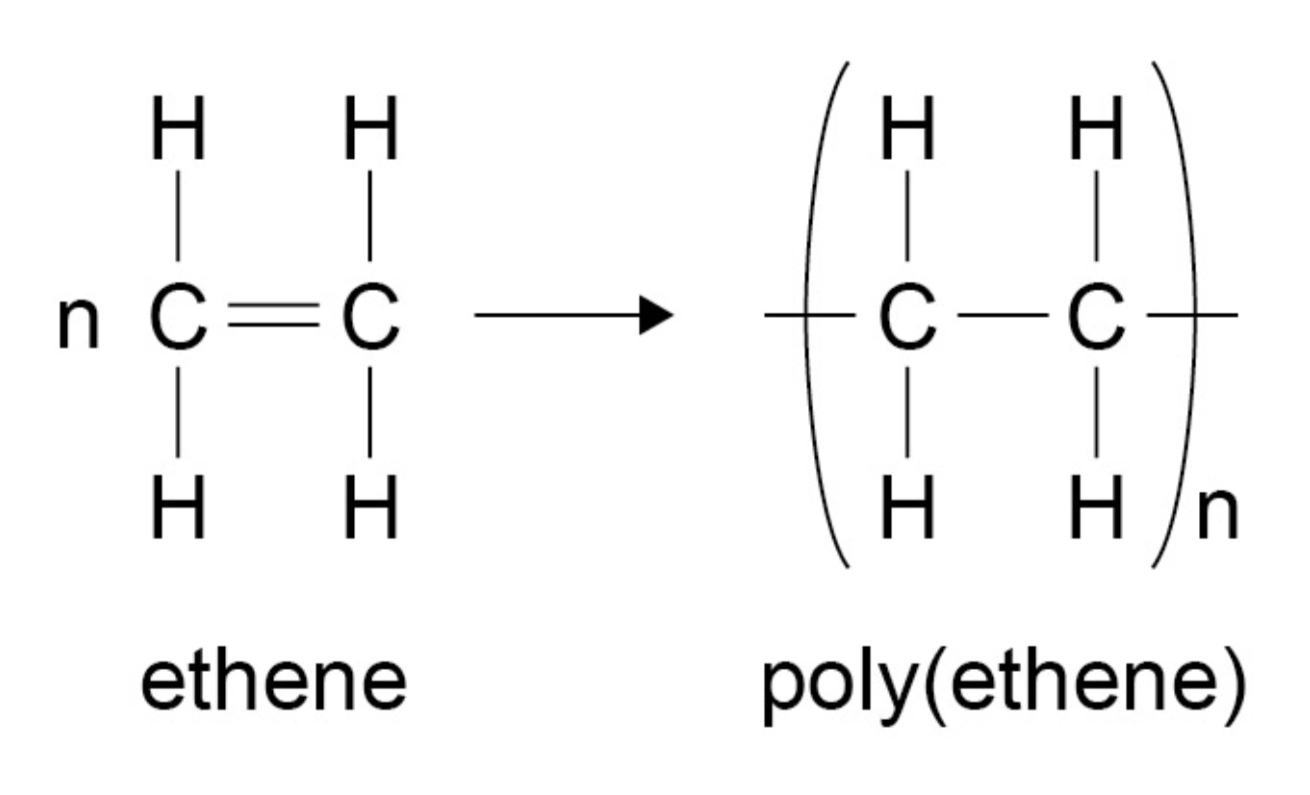
ᵀ In addition polymers, why does the repeating unit have the same atoms as the monomer?
Because no other molecule is formed in the reaction
ᵀ How can addition polymers and monomers be recognised?
The presence of the functional group C=C in the monomers
ᵀᴴ What monomers does condensation polymerisation involve?
Those with two functional groups
ᵀᴴ Why are condensation reactions called condensation reactions?
When the monomers react they join together, usually losing small molecules such as water