ib chem redox
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
32 Terms
Oxidising agent
An oxidising agent is a substance that oxidises another atom or ion by causing it to lose electrons
An oxidising agent itself gets reduced – gains electrons
Therefore, the oxidation number of the oxidising agent decreases
what makes the cell rechareabel
Reverse the redox reactions that occurred during discharge
Restore the original chemicals at each electrode
“The products do not disperse/dissolve/migrate away from the electrodes (as gas).”
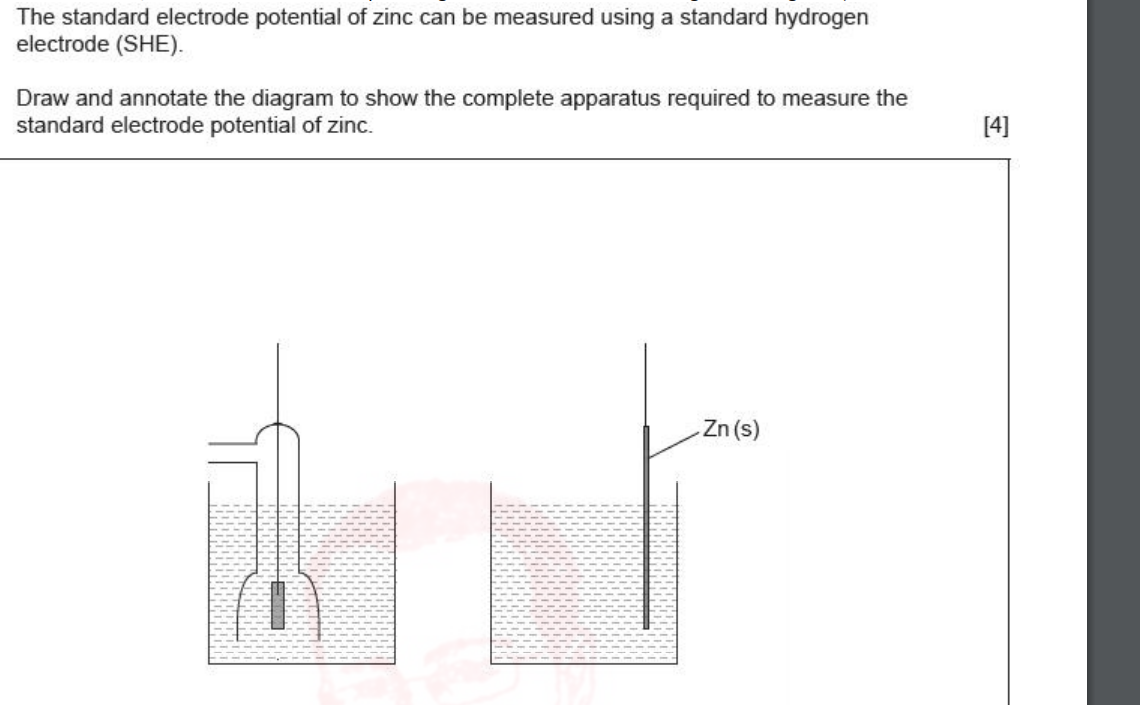
draw a diagram of apparatus to measure the standard electrode potenial of zinc
2 agents for reducing carbonyl compounds
LiAlH4, in anhydrous conditions, commonly dry ether, followed by the addition of aqueous acid
This is the stronger of these reducing agents and can reduce carboxylic acids
NaBH4, in aqueous or alcoholic solutions
This is the less hazardous of these reducing agents but it cannot reduce carboxylic acids
Both of these reagents produce the nucleophilic hydride ion, H–
electrolyte
substanced conducting electricity as liquid/aq and is chemically decomposed in the process. when its a solid it is an insulator
Reducing agent
A reducing agent is a substance that reduces another atom or ion by causing it to gain electrons
A reducing agent itself gets oxidised – loses/donates electrons
Therefore, the oxidation number of the reducing agent increases
battery
moves electyrons toward hwere they ddont wanna go - move electrons to the cathode
[o] meaning
heat with acidified potassiummdichromate
H+/Cr2O7 2-
how to oxidize primary alcohol to get an aldehyde NOT CARBOX
distillation
aldehyde have no hydrogen bonds so lower boiling point
charge carriers
in wires - electrons
in electrolyte - ions
why do we use a salt bridge
Let’s say we didn’t have a salt bridge:
Mg is losing electrons → Mg²⁺ builds up in the solution → solution becomes increasingly positive
Ag⁺ is gaining electrons → forming solid Ag → Ag⁺ concentration decreases, leaving anions behind → solution becomes increasingly negative
This charge imbalance would:
Create a voltage buildup,
Stop the reaction because the solutions resist further electron flow.
The salt bridge is filled with a neutral salt solution, like KCl or KNO₃, and allows ions (not electrons!) to move:
Anions (e.g., NO₃⁻) flow toward the anode (Mg) to balance the buildup of Mg²⁺
Cations (e.g., K⁺) flow toward the cathode (Ag) to balance the loss of Ag⁺
This keeps both sides electrically neutral, so the redox reaction can keep going.
cell notation
Anode (oxidation) | solution (highest ox state) || solution (highest ox state) | Cathode (reduction)
FUEL CELL
electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode
as fuel enters cell it becomes oxidised, setting up a potential difference (voltage) in the cell
H-O fuel cell components
A reaction chamber with separate inlets for hydrogen and oxygen gas
An outlet for the product - water
An electrolyte of aqueous sodium hydroxide
A semi-permeable membrane that separates the hydrogen and oxygen gases
The half equations are
2H2 (g) + 4OH– (aq) → 4H2O (l) + 4e– Eθ = -0.83 V
O2 (g) + 2H2O + 4e– → 4OH– (aq) Eθ = +0.40 V
The overall reaction is found by combining the two half equations and cancelling the common terms:
2H2 (g) + 4OH– (aq) + O2 (g) + 2H2O + 4e– → 4H2O (l) + 4e– + 4OH– (aq)
2H2 (g) + O2 (g) → 2H2O (l) Eθ = +1.23 V
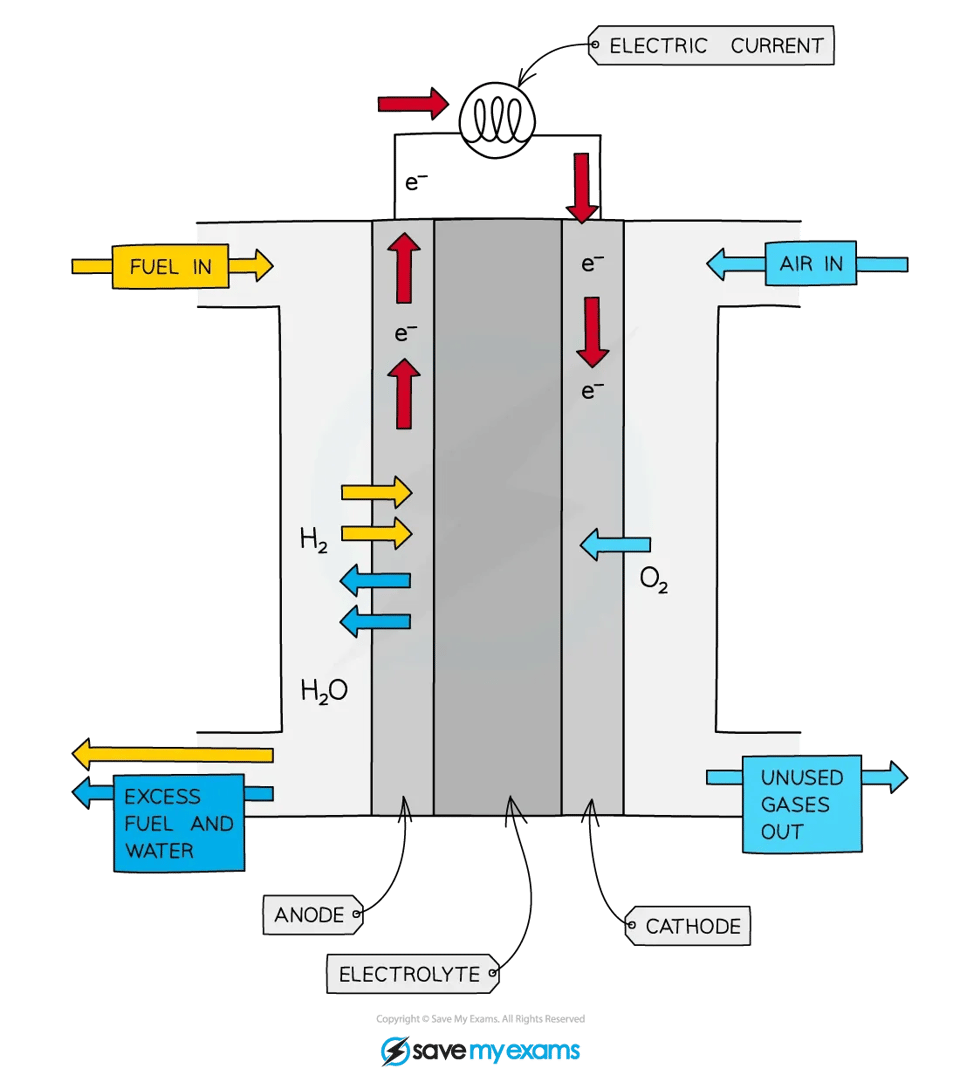
benefits of fuel cells (4)
only product is water → no pollution
The reaction is the same as hydrogen combusting in oxygen, but since the reaction takes place at room temperature without combustion, all the bond energy is converted into electrical energy instead of heat and light
No NOₓ (nitrogen oxide) emissions cuz it not in high temp
Water produced can be used on spacecraft)
1 difference between fuel and other cells
the cell operates continuously as long as there is a supply of hydrogen and oxygen
The energy is not stored in the cell
limitations of fuel cells
Hydrogen is flammable → safety risk
Storage is difficult → needs heavy, costly containers
Currently made from crude oil → non-renewable
hydrogen has High energy per gram but low energy per volume → bulky storage
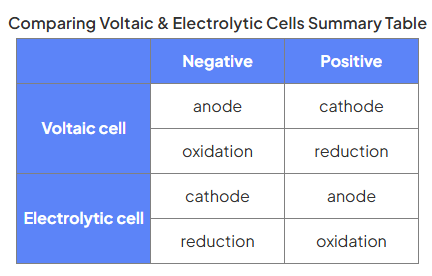
voltaic vs electrolytic cells
Voltaic cells generate electricity from chemical reactions
This is a spontaneous reaction which drives electrons around a circuit
Electrolytic cells drive chemical reactions using electrical energy
An electric current reverses the normal directions of chemical change and this is non-spontaneous
secondary (rechargeable) cell
employ chemical reactions which can be reversed by applying a voltage greater than E°cell, causing electrons to push in the opposite direction
lead-acid
NiCad
Lithium-ion
lead acid battery
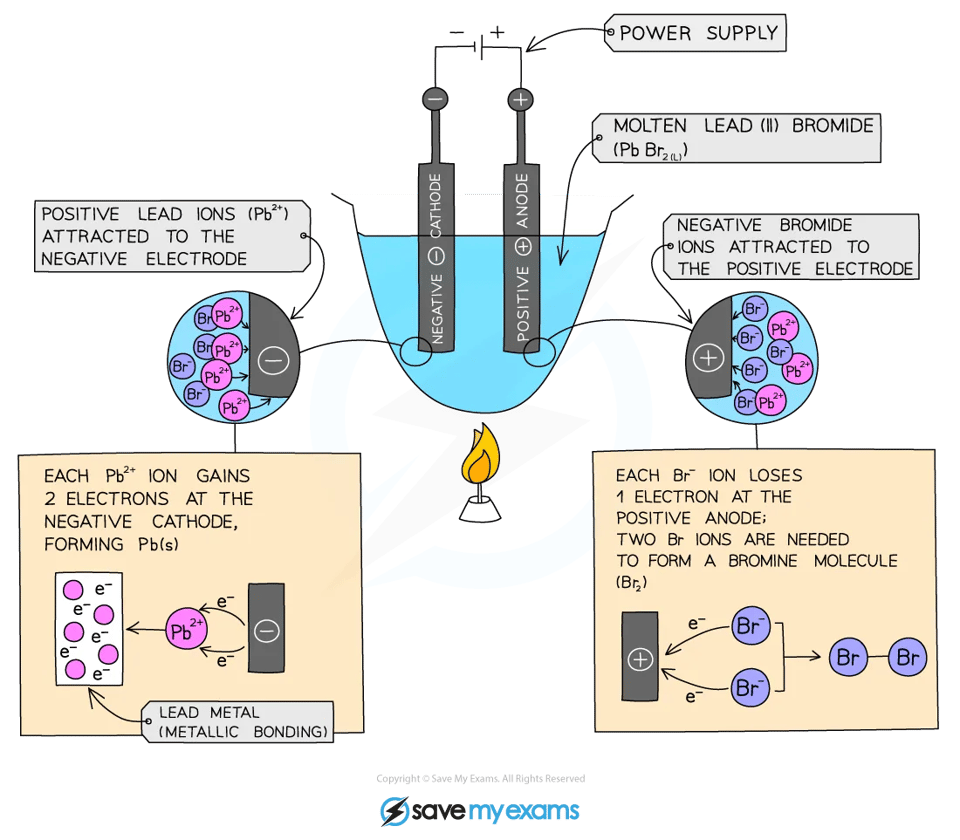
electrolytic cell
n electrolysis, the substance that the current passes through and splits up is called the electrolyte
The electrolyte contains positive and negative ions
Negative ions move to the anode and lose electrons - this is oxidation
Positive ions move to the cathode and gain electrons - this is reduction
Since metals are always cations and non-metal anions, it is easy to predict the products of electrolysis of molten salts:
Metals will always be formed at the cathode and non-metals at the anode
distillation and reflux (reboiling) of alcohol
To produce an aldehyde from a primary alcohol the reaction mixture must be heated
The aldehyde product has a lower boiling point than the alcohol ( since it has lost the H-bonding) so it can be distilled off as soon as it forms
FORMATION OF ALDEHYDE FROM CARBOXYLIC ACID
You have to use LiAlH4 refluxed in dry ether, followed by dilute acid
This reaction cannot be stopped at the aldehyde because the LiAlH4 is too powerful
To form an aldehyde from a carboxylic acid, you have to reduce the carboxylic acid down to a primary alcohol and then oxidise it back up to the aldehyde
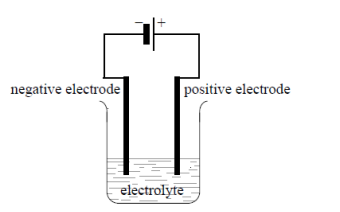
draw, using a diagram, essential components of the electrolytic cell
power supply, connecting wires, pos/neg electrode, and labelled electrolyte eg. NaCl
state one example showing economic importance of electrolysis
electroplating / purifying a metal
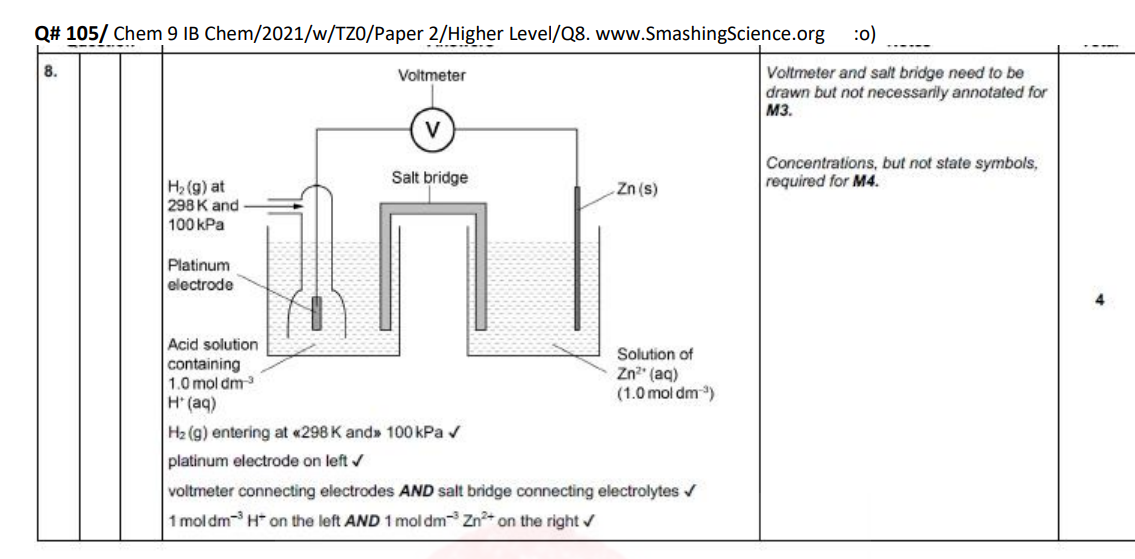
standard hydrogen electrode
Standard Hydrogen Electrode (SHE) = reference electrode at E° = 0.00 V
Involves hydrogen gas, acid solution (to be reduced, often a HCL), and an inert platinum electrode. MUST BE 1 MOL OF H
Balances: 2H⁺ + 2e⁻ ⇌ H₂
Used to measure standard electrode potentials of other half-cells by connecting and using a high-resistance voltmeter.
connecting another half cell to the SHE allows us to measure that half cells standard EP relative to SHE’s 0v
If the half-cell pushes electrons toward the SHE → its E° is negative.
If the half-cell pulls electrons from the SHE → its E° is positive.
how to find EMF (electromotive force)
EMF = E°(reduction) - E°(oxidation)
pls dont multiply them if u balance lol they are standardized
aqueous hydrolysis tip
Tables usually show standard reduction potentials (E°), so:
A higher (more positive) E° → easier to reduce (not oxidize).
A lower (more negative) E° → harder to reduce → easier to oxidize!
✅ So:
Species with lower reduction potential (more negative E°) are harder to reduce and thus easier to oxidize.
More negative E° → more likely to be oxidize
why can polyatomic ions not oxidize
polyatomic ions central atom is already at its maximum oxidation number so cant lose more electrons. water will win and get oxidized at anode
what to do at cathode vs anode at a an aqueous hydrolysis reation
At the cathode:
Always reduce the species with more positive E° (no difference if electrode is active or inert).
At the anode:
Inert electrode → oxidize water (if anion like SO₄²⁻ can't oxidize).
Active electrode → electrode itself can dissolve (like copper oxidizing into Cu²⁺).