Chapter 8-12 Vocab
0.0(0)
Card Sorting
1/149
Earn XP
Description and Tags
Last updated 2:24 AM on 12/20/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
150 Terms
1
New cards
Actinides
The Period 7 elements that constitute the second inner transition series (5*f* block), which includes thorium (Th; *Z* = 90) through lawrencium (Lr; *Z* = 103)
2
New cards
Amphoteric
Able to act as either an acid or a base
3
New cards
Atomic size
A measure of how closely one atom lies next to another, determined from the distances between nuclei of adjacent atoms.
4
New cards
Afbau principle
The conceptual approach for building up atoms by adding one proton at a time to the nucleus and one electron to the lowest energy sublevel that is available, to obtain the ground-state electron configurations of the elements.
5
New cards
Covalent radius
One-half the shortest distance between nuclei of identical covalently bonded atoms.
6
New cards
Diamagnetism
The tendency of a species not to be attracted (or to be slightly repelled) by a magnetic field as a result of its electrons being paired
7
New cards
Effective nuclear charge (Zeff)
The nuclear charge an electron actually experiences as a result of shielding effects due to the presence of other electrons
8
New cards
Electron affinity (EA)
The energy change (in kJ) accompanying the addition of 1 mol of electrons to 1 mol of gaseous atoms or ions
9
New cards
Electron configuration
The distribution of electrons within the levels and sublevels of the atoms of an element; also the notation for such a distribution
10
New cards
Hund’s rule
A principle stating that, when orbitals of equal energy are available, the electron configuration of lowest energy has the maximum number of unpaired electrons with parallel spins
11
New cards
Inner electrons (core electrons)
Electrons that fill all the energy levels of an atom except the valence level; electrons also present in atoms of the previous noble gas and any completed transition series
12
New cards
Inner transition elements
The elements of the periodic table in which the seven inner *f* orbitals are being filled; the lanthanides and the actinides
13
New cards
Ionic radius
The size of an ion as measured by the distance between the nuclei of adjacent ions in a crystalline ionic compound
14
New cards
Ionization energy (IE)
The energy (in kJ) required for complete removal of 1 mol of electrons from 1 mol of gaseous atoms or ions
15
New cards
Isoelectronic
Having the same number and configuration of electrons as another species
16
New cards
Lanthanides (also *rare earths)*
The Period 6 (4*f*) series of inner transition elements, which includes cerium (Ce; *Z* = 58) through lutetium (Lu; *Z* = 71)
17
New cards
Metallis radius
One-half the shortest distance between the nuclei of adjacent individual atoms in a crystal of an element
18
New cards
Orbital diagram
A depiction of orbital occupancy in terms of electron number and spin shown by means of arrows in a series of small boxes or on a series of short lines
19
New cards
Outer electrons
Electrons that occupy the highest energy level (highest *n* value) and are, on average, farthest from the nucleus
20
New cards
Paramagnetism
The tendency of a species with unpaired electrons to be attracted by an external magnetic field
21
New cards
Pauli exclusion principle
A principle stating that no two electrons in an atom can have the same set of four quantum numbers. The principle arises from the fact that an orbital has a maximum occupancy of two electrons and their spins are paired
22
New cards
Penetration
The phenomenon in which an outer electron moves through the region occupied by the core electrons to spend part of its time closer to the nucleus; penetration increases the average effective nuclear charge for that electron
23
New cards
Periodic law
A law stating that, when the elements are arranged by atomic mass, they exhibit a periodic recurrence of properties
24
New cards
pseudo-noble gas configuration
The (*n* − 1) *d*10 configuration of a *p*-block metal ion that has an empty outer energy level
25
New cards
Shielding (screening)
The ability of other electrons, especially those occupying inner orbitals, to lessen the nuclear attraction for an outer electron
26
New cards
Spin quantum number (ms)
A number, either 1/2 or -1/2 that indicates the direction of electron spin
27
New cards
Transition element (inner transition element)
An element that occupies the *d* block or the *f* block of the periodic table
28
New cards
Valence electrons
The electrons involved in compound formation; in main-group elements, the electrons in the outer level
29
New cards
Alloy
A mixture with metallic properties that consists of solid phases of two or more pure elements, a solid-solid solution, or distinct intermediate phases
30
New cards
Bond energy (also *bond enthalpy* or *bond strength*)
The standard enthalpy change (always > 0) accompanying the breakage of a given bond in 1 mol of gaseous molecules
31
New cards
Bond length
The distance between the nuclei of two bonded atoms
32
New cards
bond order
The number of electron pairs shared by two bonded atoms.
33
New cards
Born-Haber cycle
A series of hypothetical steps and their enthalpy changes that converts elements to an ionic compound; it is used to calculate the lattice energy.
34
New cards
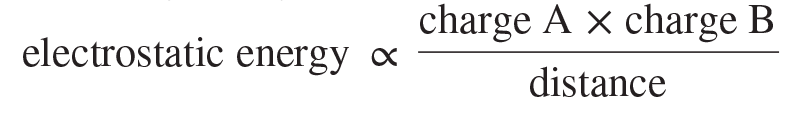
Coulomb’s law
A law stating that the electrostatic energy between particles A and B is directly proportional to the product of their charges and inversely proportional to the distance between them:
35
New cards
covalent bond
A type of bond in which atoms are bonded through the sharing of electrons; the mutual attraction of the nuclei and an electron pair that holds atoms together in a molecule.
36
New cards
covalent bonding
The idealized bonding type that is based on localized electron-pair sharing between two atoms with little difference in their tendencies to lose or gain electrons (most commonly nonmetals).
37
New cards
double bond
A covalent bond that consists of two bonding pairs; two atoms sharing four electrons in the form of one σ and one π bond.
38
New cards
electronegativity (EN)
The relative ability of a bonded atom to attract shared electrons.
39
New cards
electronegativity difference ( delta EN)
The difference in electronegativities between two bonded atoms.
40
New cards
electron-sea model
A qualitative description of metallic bonding proposing that metal atoms pool their valence electrons in a delocalized “sea” of electrons in which the metal ions (nuclei and core electrons) are submerged in an orderly array.
41
New cards
infrared (IR) spectroscopy
An instrumental technique for determining the types of bonds in a covalent molecule by measuring the absorption of IR radiation.
42
New cards
ion pairs
A gaseous ionic molecule, formed when an ionic compound vaporizes.
43
New cards
ionic bonding
The idealized type of bonding based on the attraction of oppositely charged ions that arise through electron transfer between atoms with large differences in their tendencies to lose or gain electrons (typically metals and nonmetals).
44
New cards
lattice energy ( delta H lattice)
The enthalpy change (always positive) that accompanies the separation of 1 mol of a solid ionic compound into gaseous ions.
45
New cards
Lewis electon-dot symbol
A notation in which the element symbol represents the nucleus and inner electrons and surrounding dots represent the valence electrons.
46
New cards
lone ( unshared) pair
An electron pair that is part of an atom’s valence level but not involved in covalent bonding.
47
New cards
metalllic bonding
An idealized type of bonding based on the attraction between metal ions and their delocalized valence electrons.
48
New cards
nonpolar covalent bond
A covalent bond between identical atoms in which the bonding pair is shared equally.
49
New cards
octet rule
The observation that, when atoms bond, they often lose, gain, or share electrons to attain a filled outer level of eight electrons (or two for H and Li).
50
New cards
partial ionic character
An estimate of the actual charge separation in a bond (caused by the electronegativity difference of the bonded atoms) relative to complete separation.
51
New cards
polar covalent bond
A covalent bond in which the electron pair is shared unequally, so the bond has partially negative and partially positive poles.
52
New cards
shared (bonding) pair
An electron pair shared by two nuclei; the mutual attraction between the nuclei and the electron pair forms a covalent bond.
53
New cards
single bond
A bond that consists of one electron pair.
54
New cards
triple bond
A covalent bond that consists of three bonding pairs, two atoms sharing six electrons; one σ and two π bonds.
55
New cards
axial group
An atom (or group) that lies above or below the trigonal plane of a trigonal bipyramidal molecule, or a similar structural feature in a molecule.
56
New cards
bent (V-shaped)
A molecular shape that arises when a central atom is bonded to two other atoms and has one or two lone pairs; occurs as the AX2E shape class (bond angle < 120°) in the trigonal planar arrangement and as the AX2E2 shape class (bond angle < 109.5°) in the tetrahedral arrangement.
57
New cards
bond angle
The angle formed by the bonds joining the nuclei of two surrounding atoms to the nucleus of the central atom, which is at the vertex.
58
New cards
dipole moment
A measure of molecular polarity; the magnitude of the partial charges on the ends of a molecule (in coulombs) times the distance between them (in meters).
59
New cards
electron deficient
Referring to a bonded atom, such as Be or B, that has fewer than eight valence electrons.
60
New cards
electron-pair delocalisation
The process by which electron density is spread over several atoms rather than remaining between two.
61
New cards
equatorial group
An atom (or group) that lies in the trigonal plane of a trigonal bipyramidal molecule, or a similar structural feature in a molecule.
62
New cards
expanded valence shell
A valence level that can accommodate more than eight electrons by using available *d* orbitals; occurs only with central nonmetal atoms from Period 3 or higher.
63
New cards
formal charge
The hypothetical charge on an atom in a molecule or an ion, equal to the number of valence electrons minus the sum of all the unshared and half the shared valence electrons.
64
New cards
free radical
A molecular or atomic species with one or more unpaired electrons, which typically make it very reactive.
65
New cards
Lewis structure (formula)
A structural formula consisting of electron-dot symbols, with lines as bonding pairs and dots as lone pairs.
66
New cards
linear arrangment
The geometric arrangement obtained when two electron groups maximize their separation around a central atom.
67
New cards
linear shape
A molecular shape formed by three atoms lying in a straight line, with a bond angle of 180° (shape class AX2 or AX2E3).
68
New cards
molecular polarity
The overall distribution of electronic charge in a molecule, determined by its shape and bond polarities.
69
New cards
molecular shape
The three-dimensional arrangement of the atomic nuclei in a molecule.
70
New cards
octahedral arrangment
The geometric arrangement obtained when six electron groups maximize their space around a central atom; when all six groups are bonding groups, the molecular shape is octahedral (AX6; ideal bond angle = 90°).
71
New cards
resonance hybrid
The weighted average of the resonance structures for a species.
72
New cards
resonance structure (form)
One of two or more Lewis structures for a species that cannot be adequately depicted by a single structure. Resonance structures differ only in the position of bonding and lone electron pairs.
73
New cards
seesaw shape
A molecular shape caused by the presence of one equatorial lone pair in a trigonal bipyramidal arrangement (AX4E).
74
New cards
square planar shape
A molecular shape (AX4E2) caused by the presence of two lone pairs at opposite vertices in an octahedral electron-group arrangement.
75
New cards
T-shaped
A molecular shape caused by the presence of two equatorial lone pairs in a trigonal bipyramidal arrangement (AX3E2).
76
New cards
tetrahedral arrangement
The geometric arrangement formed when four electron groups maximize their separation around a central atom; when all four groups are bonding groups, the molecular shape is tetrahedral (AX4; ideal bond angle 109.5°).
77
New cards
trigonal bipyramidal arrangement
The geometric arrangement formed when five electron groups maximize their separation around a central atom. When all five groups are bonding groups, the molecular shape is trigonal bipyramidal (AX5; ideal bond angles, axial-center-equatorial 90° and equatorial-center-equatorial 120°).
78
New cards
trigonal planar arrangement
The geometric arrangement formed when three electron groups maximize their separation around a central atom. When all three groups are bonding groups, the molecular shape is trigonal planar (AX3; ideal bond angle 120°).
79
New cards
trigonal pyramidal arrangement
A molecular shape (AX3E) caused by the presence of one lone pair in a tetrahedral arrangement.
80
New cards
valence-shell electron-pair repulsion (VSEPR) theory
A model explaining that the shapes of molecules and ions result from minimizing electron-pair repulsions around a central atom.
81
New cards
square pyramidal shape
A molecular shape (AX5E) caused by the presence of one lone pair in an octahedral electron-group arrangement.
82
New cards
amorphous solid
A solid that has a poorly defined shape because its particles do not have an orderly arrangement throughout a sample.
83
New cards
atomic solid
A solid consisting of individual atoms held together by dispersion forces; the frozen noble gases are the only examples.
84
New cards
band theory
An extension of molecular orbital (MO) theory that explains many properties of metals and other solids—in particular, the differences in conductivity of metals, metalloids, and nonmetals.
85
New cards
body-centered cubic unit cell
A unit cell in which a particle lies at each corner and in the center of a cube.
86
New cards
boiling point
The temperature at which the vapor pressure inside bubbles forming in a liquid equals the external (atmospheric) pressure.
87
New cards
branch
A side chain appended to a polymer backbone or to the longest sequence of atoms in an organic compound.
88
New cards
capillarity (capillary action)
The rising of a liquid through a narrow space against the pull of gravity.
89
New cards
ceramic
A nonmetallic, nonpolymeric solid that is hardened by heating it to high temperatures and, in most cases, consists of silicate microcrystals suspended in a glassy cementing medium.
90
New cards
**Clausius-Clapeyron equation**
An equation that expresses the linear relationship between vapor pressure *P* of a liquid and temperature *T*; in two-point form, it is
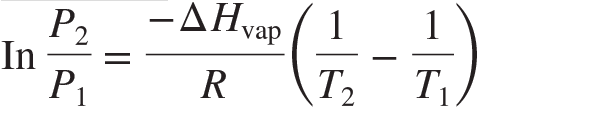
91
New cards
condensation
The process of a gas changing into a liquid.
92
New cards
conduction band
In band theory, the empty, higher energy portion of the band of molecular orbitals into which electrons move when conducting heat and electricity.
93
New cards
conductor
A substance (usually a metal) that conducts an electric current well.
94
New cards
coordination number
In a crystal, the number of nearest neighbors surrounding a particle. In a complex ion, the number of ligand atoms bonded to the central metal ion.
95
New cards
copolymer
A polymer that consists of two or more types of monomer.
96
New cards
critical point
The point on a phase diagram above which the vapor cannot be condensed to a liquid; the end of the liquid-gas curve.
97
New cards
crosslink
A branch that covalently joins one polymer chain to another.
98
New cards
crystal defect
Any of a variety of disruptions in the regularity of a crystal structure.
99
New cards
crystalline solid
A solid with a well-defined shape because of the orderly arrangement of the atoms, molecules, or ions.
100
New cards
cubic closet packing
A crystal structure based on the face-centered cubic unit cell in which the layers have an *abcabc* . . . pattern.