Lewis Acids and Bases
0.0(0)
0.0(0)
Card Sorting
1/13
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
1
New cards
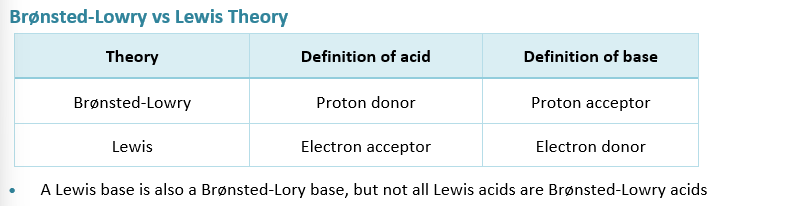
WHat is a Lewis Acid and base
Lewis acid ACCEPTS a pair of electrons
Lewis base DONATES pair of electrons
2
New cards
Why aren’t all lewis acids also Bronsted-Lowry acids
This is because a Lewis Acid also accounts for acids that cannot donate H+ ions since they don’t have one
These acids cannot classify as bronsted lowry acids though
3
New cards
What are Electrophiles?
Electron-rich species that donates lone pairs to form a covalent bond
Lewis Bases
4
New cards
What are Nucleophiles
Electron deficient species that can accept lone pairs to form covalent bond
Lewis Acid
5
New cards
6
New cards
7
New cards
8
New cards
9
New cards
10
New cards
11
New cards
12
New cards
13
New cards
14
New cards