BIOL 251 Exam 4 Environmental Microbiology
1/60
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
61 Terms
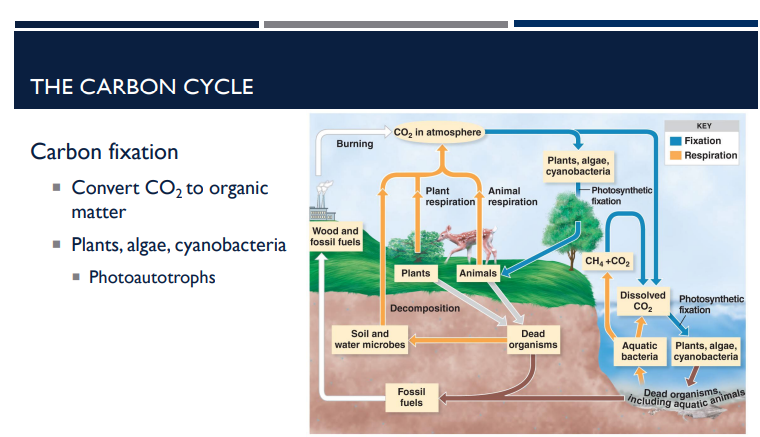
What is carbon fixation?
Carbon fixation is the process by which certain organisms take carbon dioxide (CO₂) from the atmosphere and convert it into organic molecules (like sugars). These organic molecules can then be used for energy or to build cellular structures.
Plants, algae, and cyanobacteria are organisms that are photoautotrophs. They likely perform what? (Hint: photoautotrophs use light energy to make their own organic matter from CO₂.)
carbon fixation
What is the starting point of the carbon cycle?
Carbon fixation
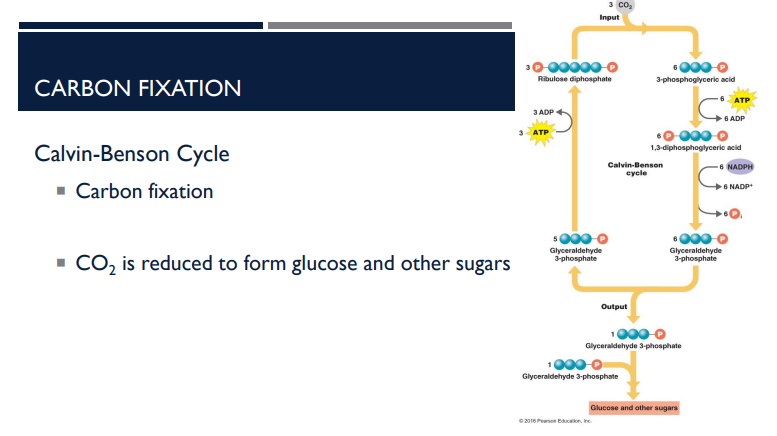
Describe the basic steps of the Calvin-Benson Cycle.
Carbon fixation (CO₂ attached to RuBP)
CO₂ is reduced (using ATP + NADPH)
Produces G3P, which can form glucose and other sugars
(LC) Where does the energy for carbon fixation come from?
Glycolysis
Fermentation
Oxidative phosphorylation
Sunlight
CO2
D
Sunlight excites electrons. These excited electrons travel down the electron transport chain, which drives th production of ATP. This ATP can then be used to fix carbon through the Calvin-Benson cycle. Thus, the energy for carbon fixation comes from sunlight.
What are chemoheterotrophs?
Get energy from chemicals (not light) → “chemo”
Get carbon from organic molecules made by other organisms → “heterotroph”
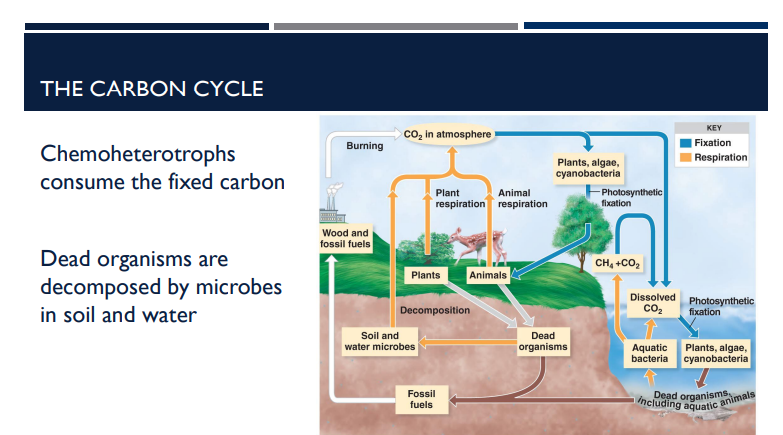
Chemoheterotrophs cannot fix CO₂ into their own organic molecules like plants do.
Instead, what do they do?
they consume organic carbon that is already made (fixed) by autotrophs.
This means they eat organic molecules (like sugars, lipids, proteins) that originally came from carbon fixation done by plants, algae, or cyanobacteria.
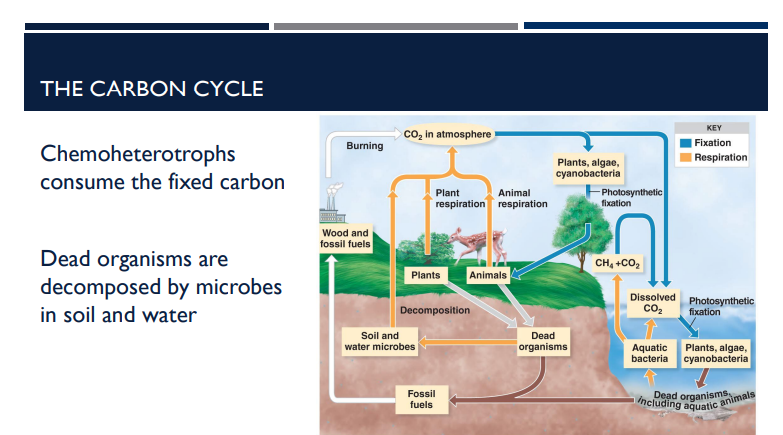
What happens when organisms die in relation to the Carbon Cycle?
Dead organisms are decomposed by microbes in soil and water.
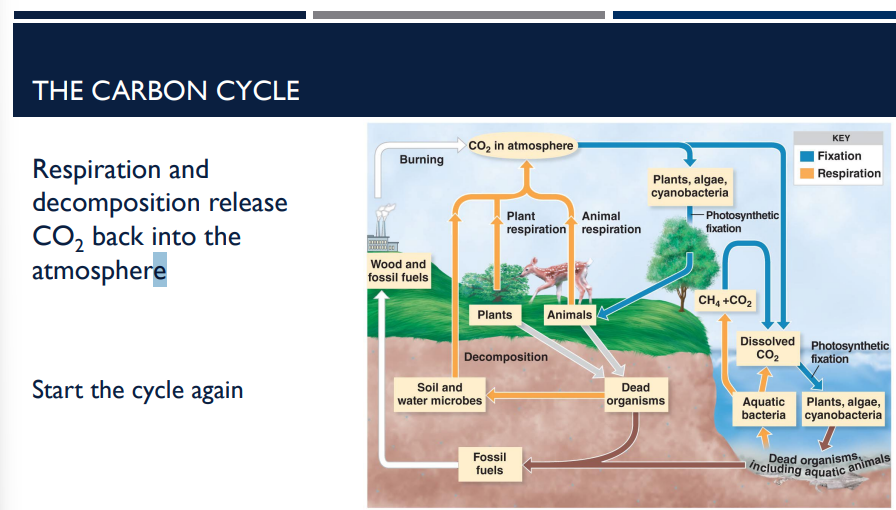
What step makes the carbon cycle start again?
Respiration and decomposition release CO2 back into the atmosphere
(LC) During respiration, where is CO2 produced?
Glycolysis
Linking reaction
Krebs cycle
Electron transport chain
Chemiosomosis
Calvin-Benson cycle
Alcohol fermentation
Lactic acid fermentation
B and C
During respiration, carbon dioxide is produced during the linking reaction and the Krebs cycle. Remember to count your carbons!
While carbon dioxide is produced during alcohol fermentation, this is not part of respiration.
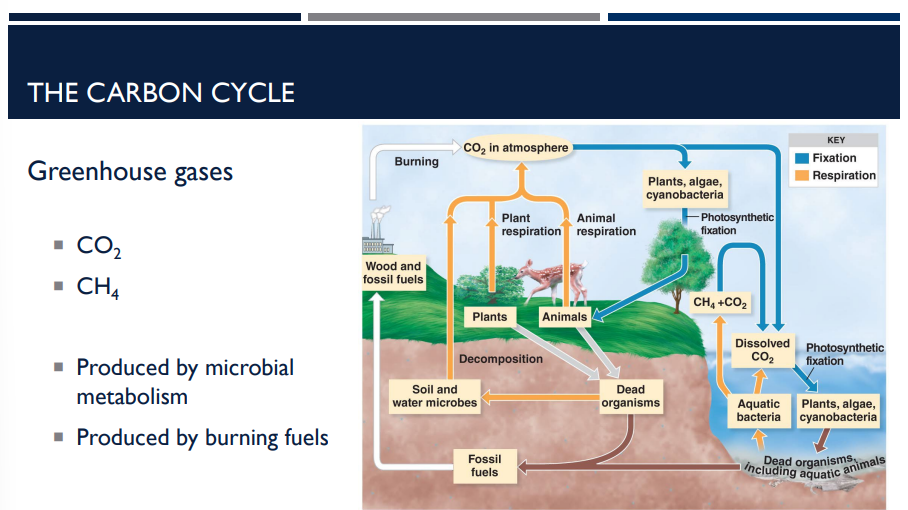
What are the two major greenhouse gases? How are they produced?
CO2 and CH4
Produced by microbial metabolism (Microbes create both CO₂ and CH₄ as natural byproducts of their metabolism)
Produced by burning fuels (Humans add large amounts of CO₂ (and some CH₄) to the atmosphere by burning:
Coal
Oil
Natural gas
Biomass)
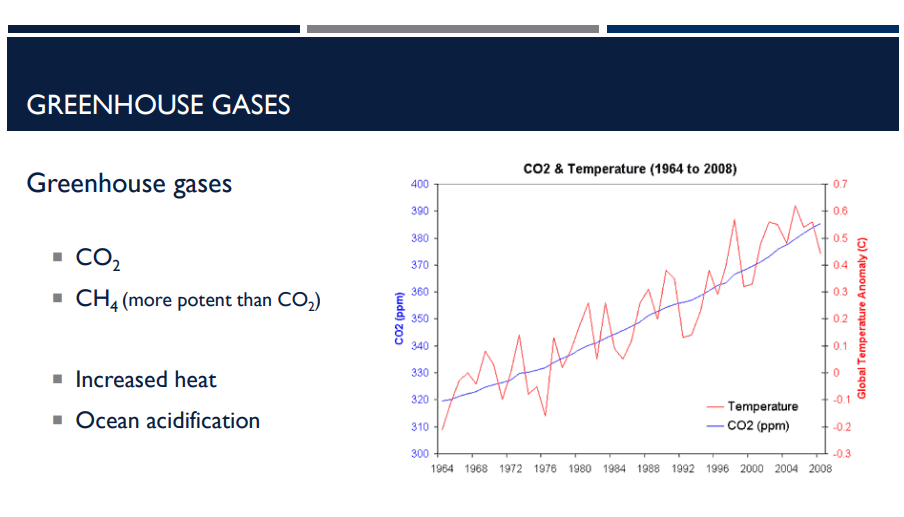
Which greenhouse gas is more potent than the other, CO2 or CH4? What does it mean to be more potent?
CH4; Methane is much stronger at trapping heat — about 25–30 times more potent than CO₂ over 100 years.
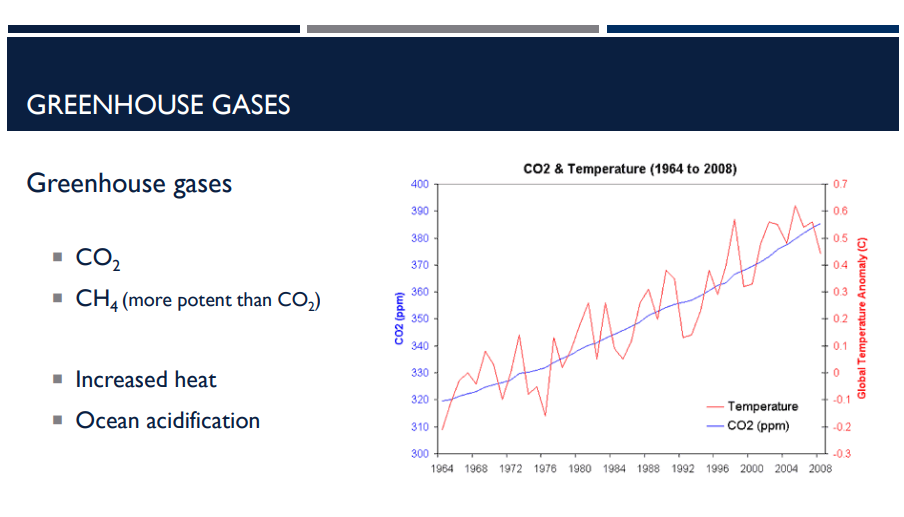
When greenhouse gases accumulate in the atmosphere, what happens?
Increased Heat
they trap more of the sun’s heat.
Ocean Acidification
Extra CO₂ in the atmosphere dissolves into ocean water.
When CO₂ mixes with seawater, it forms carbonic acid, which lowers ocean pH.

Corals are made of many tiny ____
polyps (Coral polyps are tiny, soft-bodied animals related to jellyfish and sea anemones that form colonies to build massive reefs.)

Corals are made of many tiny polyps (Coral polyps are tiny, soft-bodied animals related to jellyfish and sea anemones that form colonies to build massive reefs).
Who do they have a symbiotic relationship with?
Coral polyps have a symbiotic relationship with Zooxanthellae.
Zooxanthellae are photosynthetic algae that live inside coral polyps. They get a safe place to live inside the coral. They receive nutrients from the coral’s waste products. Zooxanthellae perform photosynthesis and provide the coral with Oxygen, Sugars/energy. This energy helps the coral grow and build its skeleton. Zooxanthellae also give corals their beautiful colors.

Rising ocean temperatures cause the coral to eject the ____
Zooxanthellae

When ocean water gets too warm (even by just 1–2°C above normal), corals become stressed. ☹
What happens?
Rising ocean temperatures cause the coral to eject the Zooxanthellae. When zooxanthellae are expelled, coral bleaching occurs where:
The coral’s white calcium carbonate skeleton shows through.
The coral appears bleached (white).

What is coral bleaching?
When ocean water gets too warm (even by just 1–2°C above normal), corals become stressed and eject the Zooxanthellae. ☹
When zooxanthellae are expelled, coral bleaching occurs where:
The coral’s white calcium carbonate skeleton shows through.
The coral appears bleached (white).
(LC) Why is nitrogen often a limiting plant nutrient, despite the fact that nearly 80% of the atmosphere is nitrogen gas (N2)?
Because plants cannot fix N2
Because plants can not assimilate nitrogen-containing compounds
Because N2 easily leaches away from soil
A
The majority of nitrogen in the atmosphere exists as N2. The two nitrogen atoms are linked by a triple bond, which is very difficult to break. Thus, most nitrogen in the atmosphere is unavailable to living organisms. Specialized organisms able to fix nitrogen into organic compounds are necessary to make the nitrogen accessible to other organisms.
What Is the Nitrogen Cycle?
The nitrogen cycle is the process that moves nitrogen between the atmosphere, soil, living organisms, and back again.
Nitrogen is essential for life (proteins, DNA), but most organisms cannot use nitrogen in its atmospheric form (N₂ gas).
The nitrogen cycle solves that problem by converting nitrogen into different usable forms.
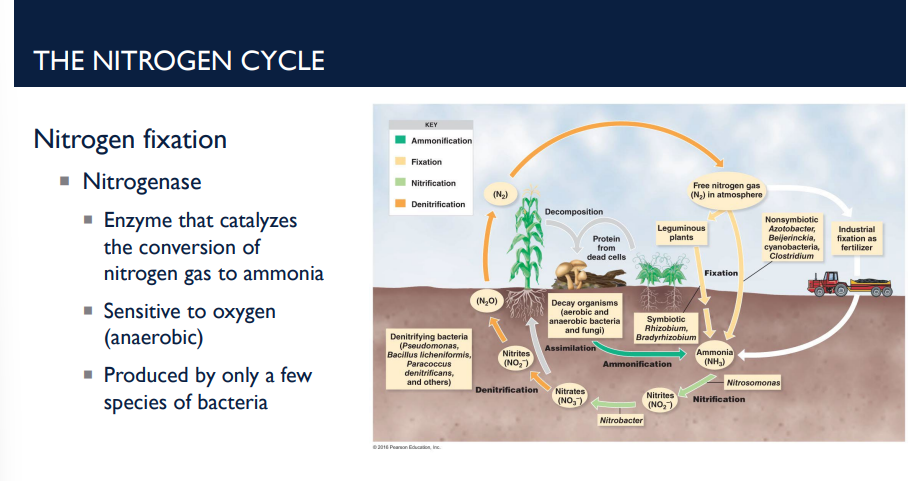
Describe the first step of the nitrogen cycle, nitrogen fixation.
Atmospheric N₂ gas → ammonia (NH₃)
Done by the enzyme nitrogenase (produced by only a few species of bacteria)
Nitrogenase works only in anaerobic (low oxygen) conditions, meaning it is sensitive to oxygen (anaerobic)
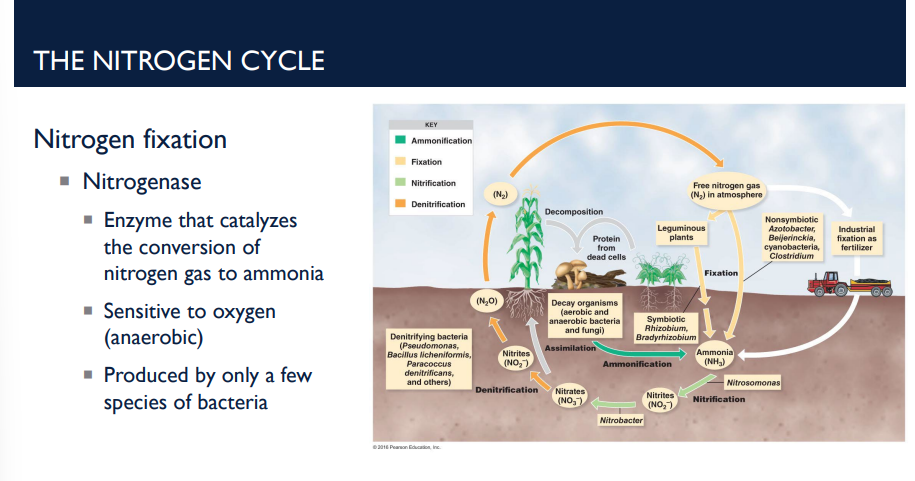
Describe what the enzyme nitrogenase does in nitrogen fixation (first step of the nitrogen cycle.
Atmospheric N₂ gas → ammonia (NH₃)
Done by the enzyme nitrogenase (produced by only a few species of bacteria)
Nitrogenase works only in anaerobic (low oxygen) conditions, meaning it is sensitive to oxygen (anaerobic)
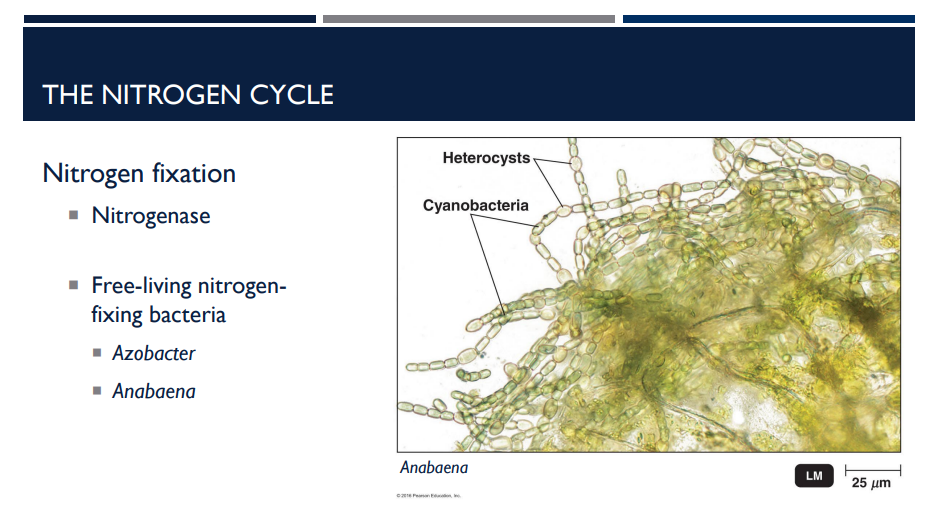
What do Free-Living Nitrogen-Fixing Bacteria do? 🦠🦠🦠🦠🦠🦠🦠🦠🦠 What are some examples?
These bacteria fix nitrogen on their own, not inside plant roots.
Azotobacter: A free-living, aerobic soil bacterium.
Anabaena: A cyanobacterium (photosynthetic, aquatic).
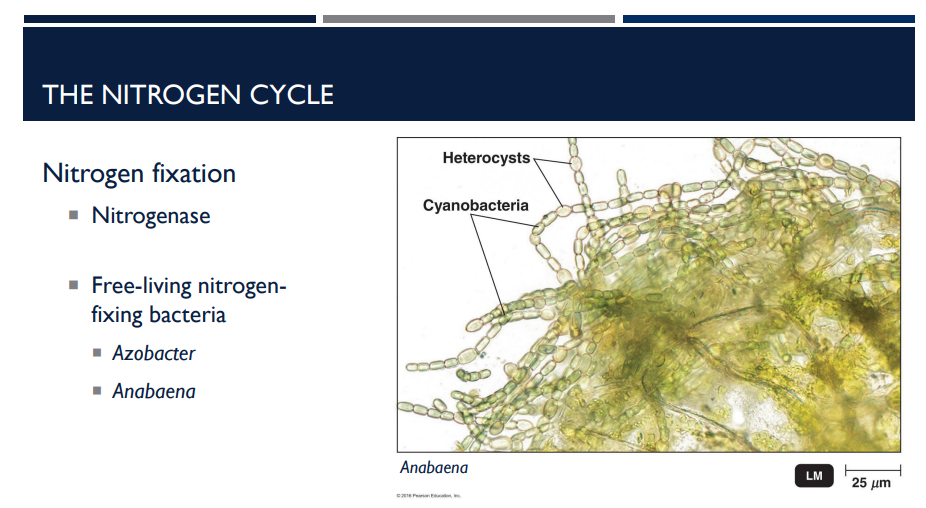
Azotobacter and anabaena are what type of bacteria?
Free-living nitrogen fixing bacteria.
These bacteria fix nitrogen on their own, not inside plant roots.
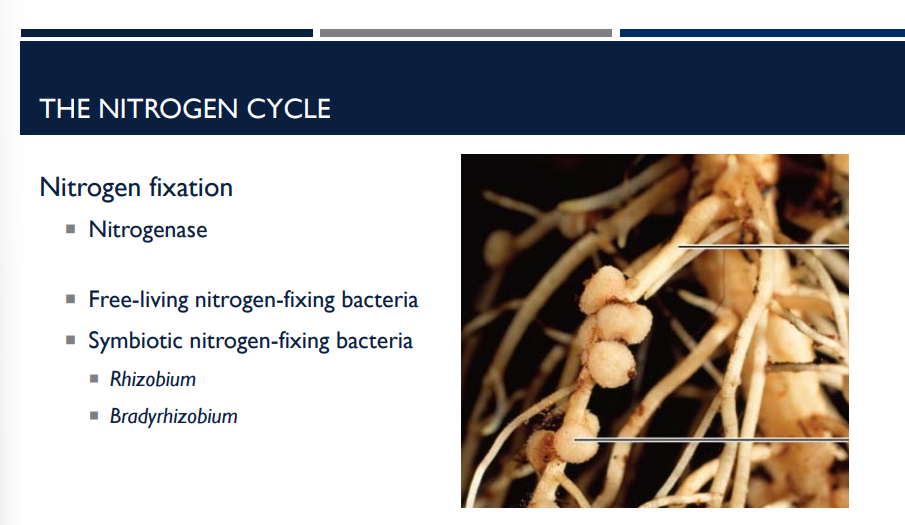
What do Symbiotic nitrogen-fixing bacteria do? 🦠🦠🦠🦠🦠🦠🦠🦠🦠 What are some examples?
These bacteria cannot fix nitrogen as efficiently on their own, so they form mutualistic symbiotic relationships with plants—both partners benefit.
Rhizobium
Bradyrhizobium
Lichens with cyanobacteria (blue-green algae)
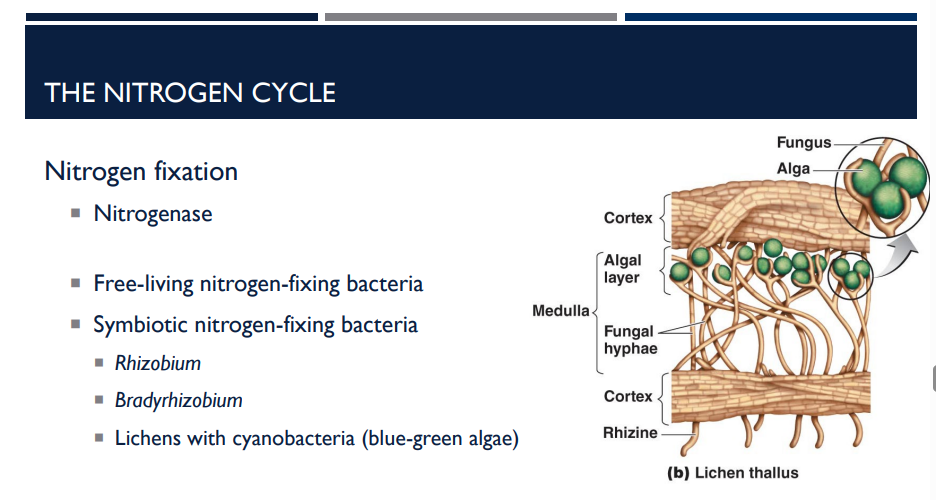
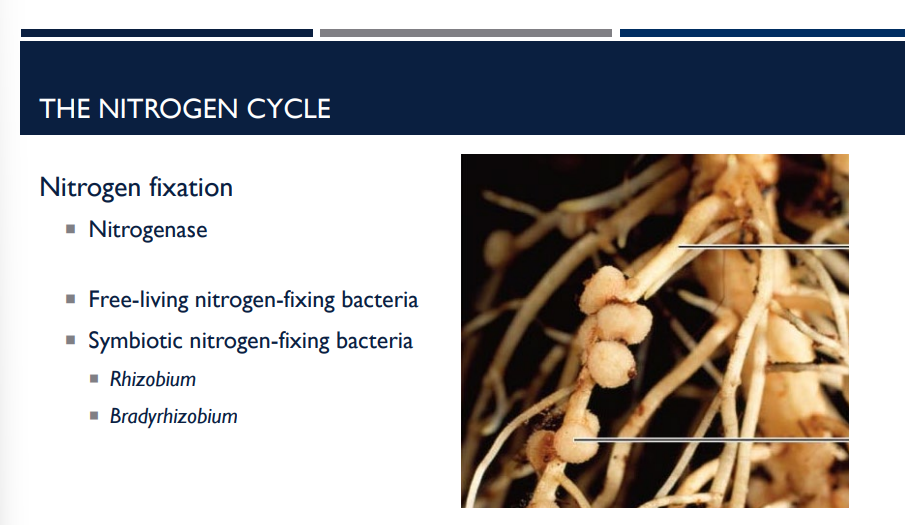
Rhizobium, Lichens with cyanobacteria (blue-green algae) & Bradyrhizobium are what type of bacteria?
Symbiotic nitrogen-fixing bacteria
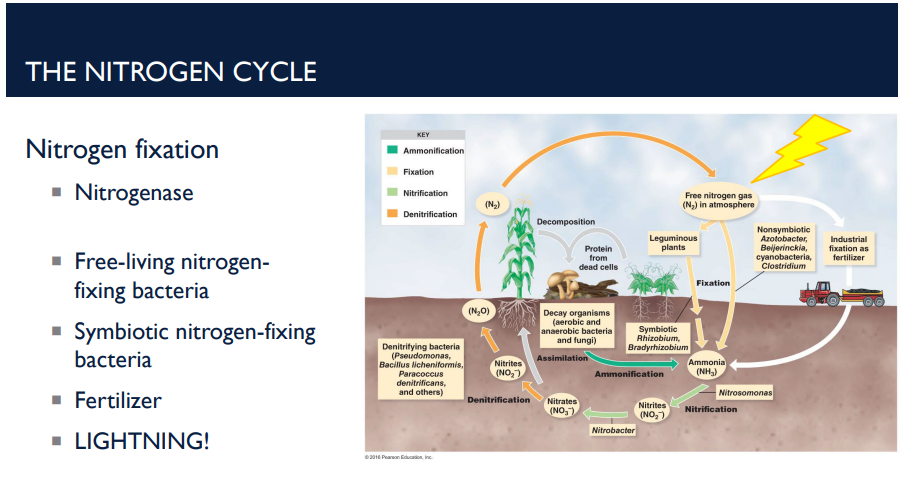
The nitrogen cycle is how nitrogen moves between the atmosphere, soil, living organisms, and back again. Humans add nitrogen to soil through synthetic ______ (like ammonium nitrate). These provide ammonia, nitrites, or nitrates directly to plants.
fertilizers
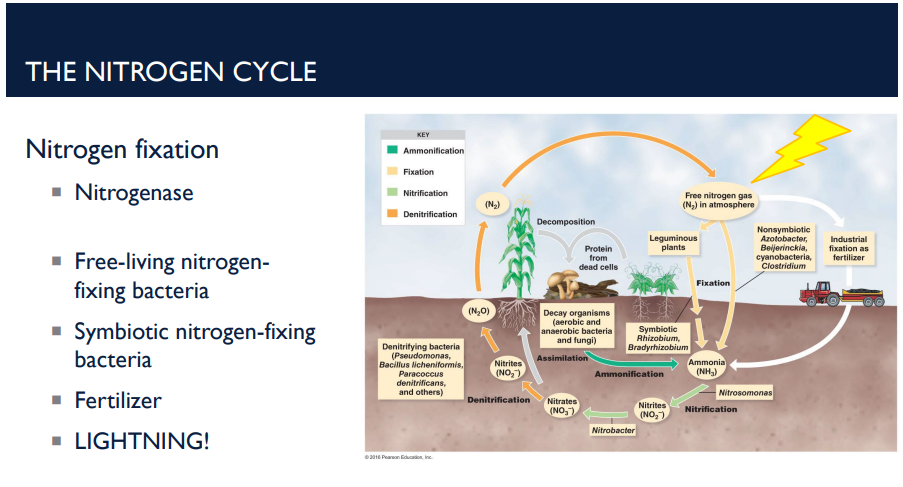
T/F: Lightning⚡⚡⚡⚡⚡⚡ is part of the nitrogen cycle.
T. The huge amount of energy in lightning splits N₂ gas. This allows nitrogen to combine with oxygen → forming nitrates. These nitrates dissolve in rainwater and enter the soil. Plants can use these nitrates just like any other nitrogen source.
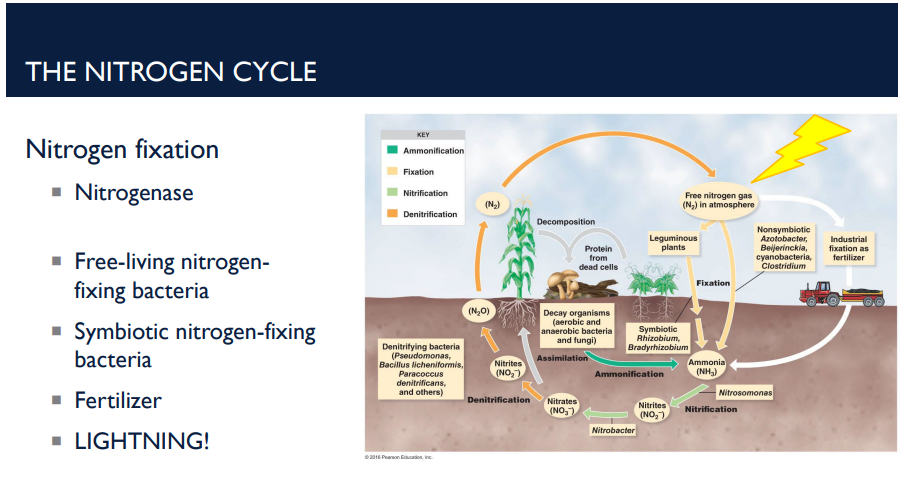
How does lightning play a role in the nitrogen cycle?
The huge amount of energy in lightning splits N₂ gas. This allows nitrogen to combine with oxygen → forming nitrates. These nitrates dissolve in rainwater and enter the soil. Plants can use these nitrates just like any other nitrogen source.
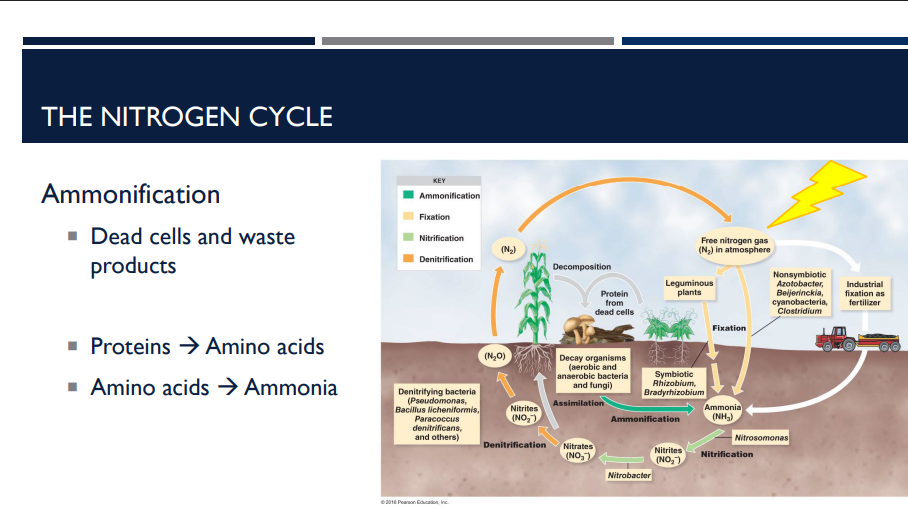
What is ammonification? Describe how it works in the nitrogen cycle.
Ammonification is a step in the nitrogen cycle where organic nitrogen from dead organisms and waste products is converted into ammonia (NH₃).
Dead cells and waste products
Plants, animals, and microbes die or excrete nitrogen-rich wastes (like urine or feces).
Proteins → Amino acids
Decomposer bacteria and fungi break down proteins in these dead/waste materials into amino acids.
Amino acids → Ammonia (NH₃)
Further microbial decomposition converts amino acids into ammonia, which goes into the soil.
This ammonia can then be used by plants or further processed in the nitrogen cycle (e.g., nitrification).
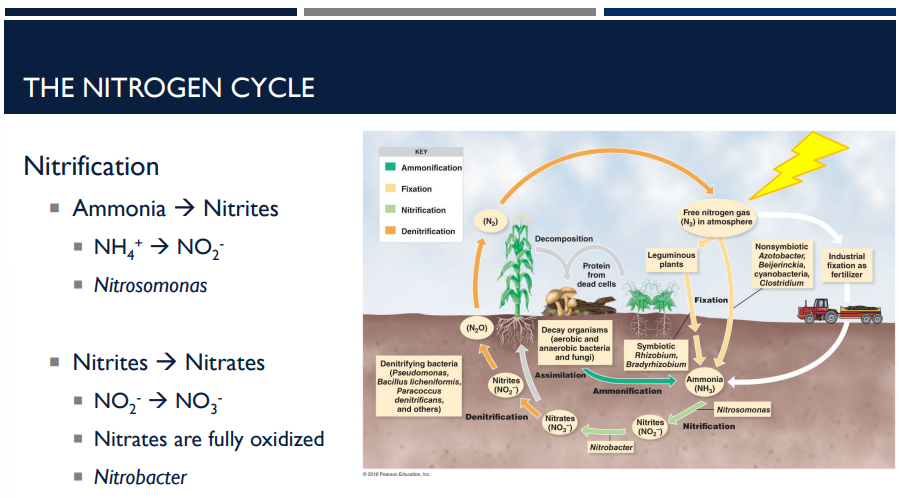
What is nitrification? Describe how it works in the nitrogen cycle.
Nitrification is the process where ammonia (NH₃) from ammonification is converted into nitrites (NO₂⁻) and then nitrates (NO₃⁻) — forms that plants can readily absorb. It’s carried out by special soil bacteria and occurs in two steps.
Step 1: Ammonia → Nitrites
Reaction: NH₄⁺ → NO₂⁻
Done by bacteria like Nitrosomonas
Ammonia is partially oxidized to nitrite
This is an energy-producing process for the bacteria
Step 2: Nitrites → Nitrates
Reaction: NO₂⁻ → NO₃⁻
Done by bacteria like Nitrobacter
Nitrites are fully oxidized to nitrates
Nitrates (NO₃⁻) are the form most easily absorbed by plants
List all the steps of the nitrogen cycle (no need to describe them). ***check later please
nitrogen fixation
nitrification
assimilation
ammonification
denitrification.
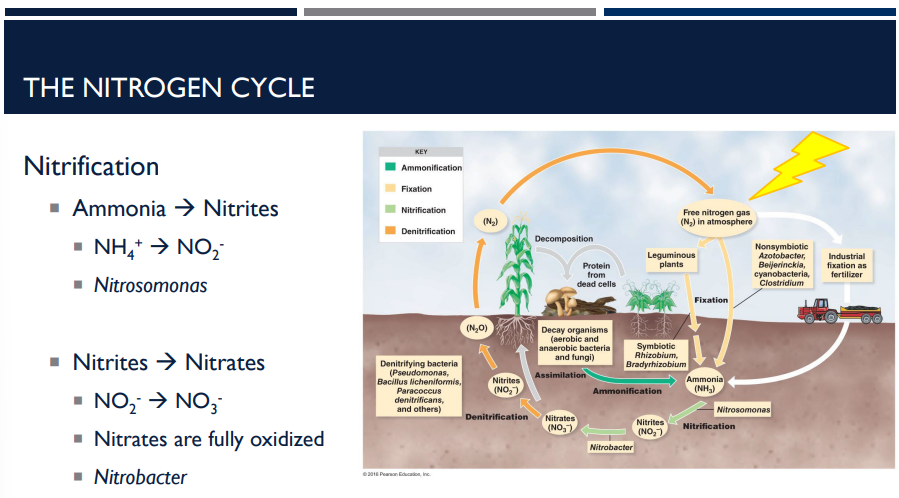
The bacteria nitrosomonas helps with a step in nitrification in the nitrogen cycle. What does it do?
Reaction: NH₄⁺ → NO₂⁻
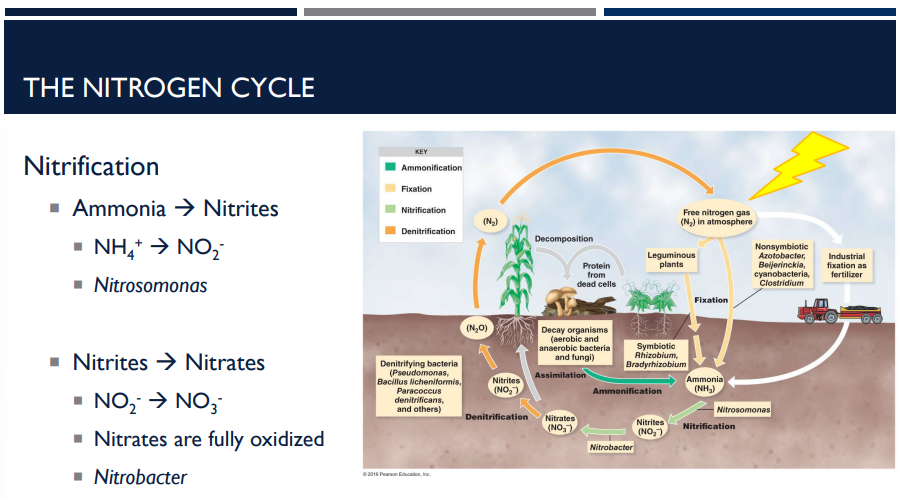
The bacteria nitrobacter helps with a step in nitrification in the nitrogen cycle. What does it do?
Reaction: NO₂⁻ → NO₃⁻. Nitrites are fully oxidized to nitrates.
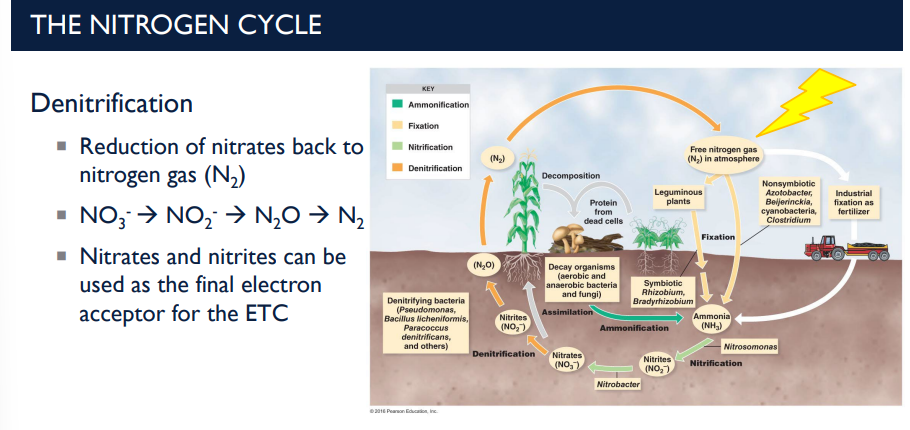
Describe the deinitrifcation step in the nitrogen cycle.
Denitrification is the process that returns nitrogen to the atmosphere by converting nitrates and nitrites back into nitrogen gas (N₂) (Reduction of nitrates back to nitrogen gas). This is mainly done by anaerobic bacteria in oxygen-poor soils or waterlogged environments.
Nitrates → Nitrites → Nitrous Oxide → Nitrogen Gas
Reaction: NO₃⁻ → NO₂⁻ → N₂O → N₂
N₂ then goes back into the atmosphere, completing the nitrogen cycle.
Common denitrifying bacteria include: Pseudomonas, Bacillus, Paracoccus (These bacteria often thrive in anaerobic or low-oxygen soils)
Electron Acceptors in the ETC
In oxygen-poor conditions, some bacteria use nitrates or nitrites as the final electron acceptor instead of oxygen.
This is why denitrification happens mostly in anaerobic environments.
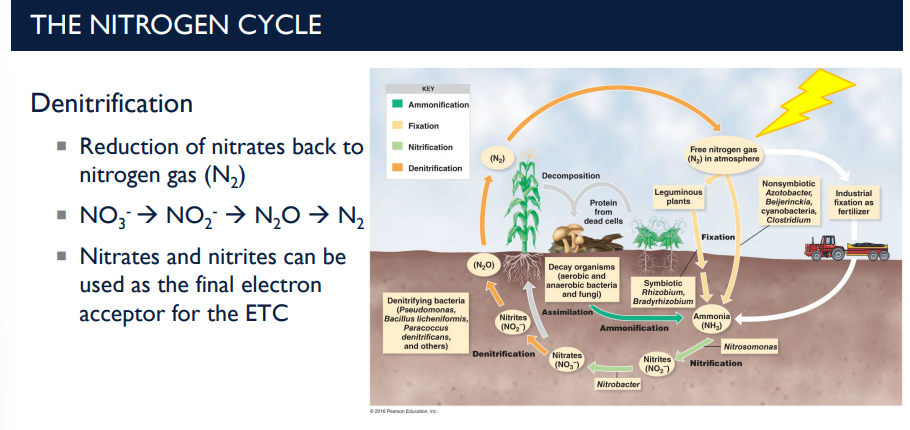
In oxygen-poor conditions, some bacteria use nitrates or nitrites as the final electron acceptor instead of ____.
oxygen. This is why denitrification happens mostly in anaerobic environments.
(LC) Name the process that uses nitrate as an electron acceptor.
Glycolysis
Ferementation
Aerobic respiration
Anaerobic respiration
Photosynthesis
D
In aerobic respiration, oxygen is used as the final electron acceptor at the end of the electron transport chain.
In anaerobic respiration, another molecule (such as nitrate) is used as the final electron acceptor.

Common denitrifying bacteria include
Pseudomonas
Bacillus
Paracoccus
These bacteria often thrive in anaerobic or low-oxygen soils, where they use nitrates/nitrites as the final electron acceptor instead of oxygen.
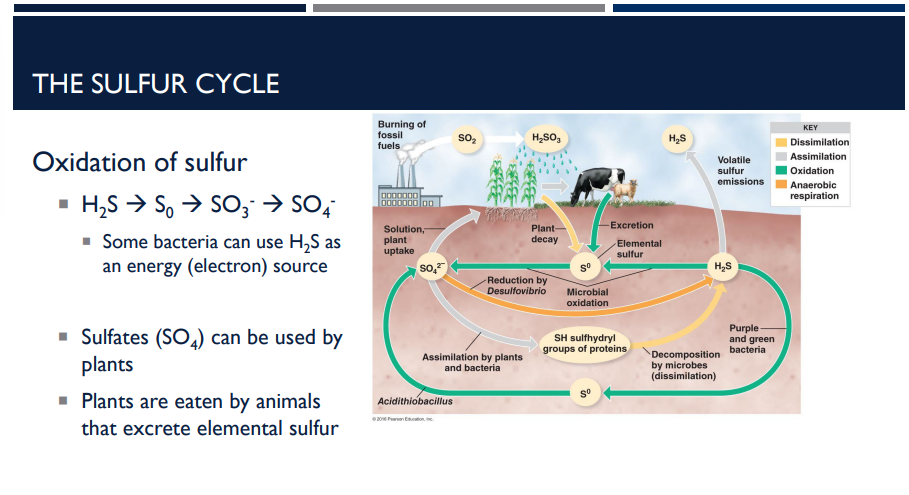
Describe the purpose of the sulfur cycle.
The sulfur cycle is how sulfur moves through the environment, living organisms, and back. Sulfur is essential for proteins and vitamins.
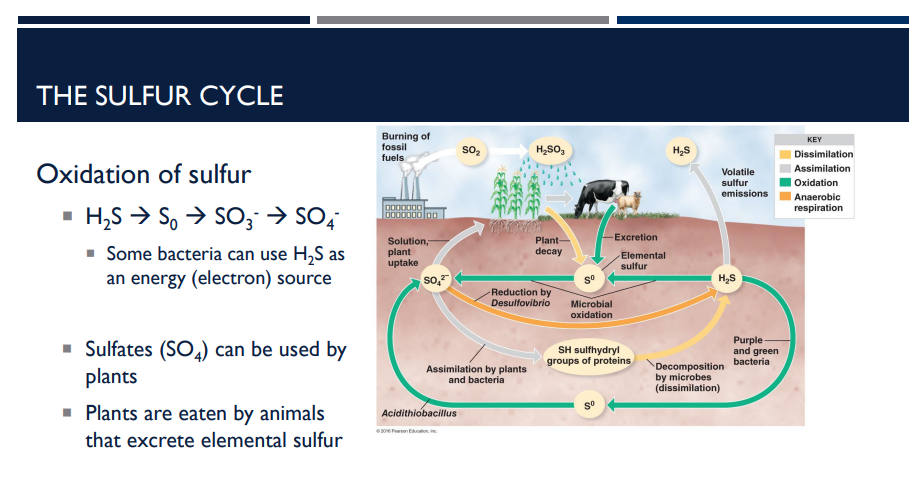
Describe the first step of the sulfur cycle, the oxidation of sulfur.
1. Oxidation of Sulfur
Sulfur changes form as it moves through the cycle:
H₂S → S⁰ → SO₃²⁻ → SO₄²⁻
Some bacteria can use H2S as an energy (electron source).
Sulfates (SO4) can be used by plants.
Plants are eaten by animals that excrete elemental sulfur.
Green and purple sulfur bacteria use H2S to fix carbon.
Acidithiobacillus converts S0 to sulfate (SO4)
GPT Context: Some bacteria, called sulfur-oxidizing bacteria, can use H₂S as an energy source, much like plants use sunlight (they transfer electrons from H₂S).

In the oxidation of sulfur, Green and purple sulfur bacteria use ___ to fix carbon
H2S

(LC) What type of organisms are green and purple sulfur bacteria?
Phototrophs
Chemotrophs
Heterotrophs
Autotrophs
A and D
Green and purple sulfur bacteria are phototrophs because they use light as a source of energy.
Green and purple sulfur bacteria are autotrophs because they use carbon dioxide as a carbon source.
They contribute to both the sulfur cycle (by oxidizing H₂S) and the carbon cycle (by fixing CO₂).
They do not release O₂, unlike oxygenic photosynthesis in plants or cyanobacteria.
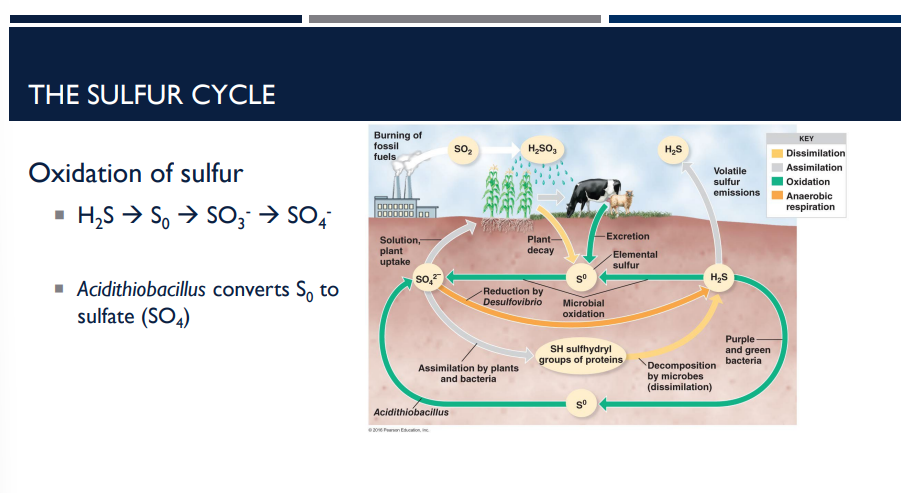
In the oxidation of sulfur, for the sulfur cycle, which bacteria convert S0 to sulfate (SO4)?
Acidithiobacillus

Describe the reduction of sulfur in the sulfur cycle.
The reduction side of the sulfur cycle is basically the reverse of oxidation. Sulfur compounds are converted back into hydrogen sulfide (H₂S) by certain bacteria and decomposition processes.
Step-by-Step Reduction
SO₄²⁻ → SO₃²⁻ → S⁰ → H₂S
This H₂S can then re-enter the oxidation cycle, creating a continuous redox cycle of sulfur. (Cycle of oxidation and reduction of sulfur)
Sulfur is also reduced during decomposition
Key bacteria player: Desulfovibrio (type of anaerobic bacteria that reduces sulfate to H₂S in the reduction of sulfur)
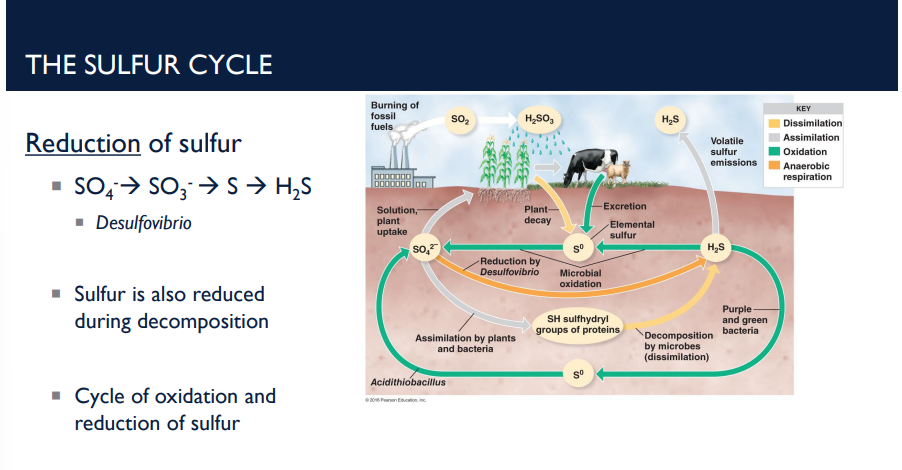
What is the type of anaerobic bacteria that reduces sulfate to H₂S in the reduction of sulfur in the sulfur cycle?
Desulfovibrio
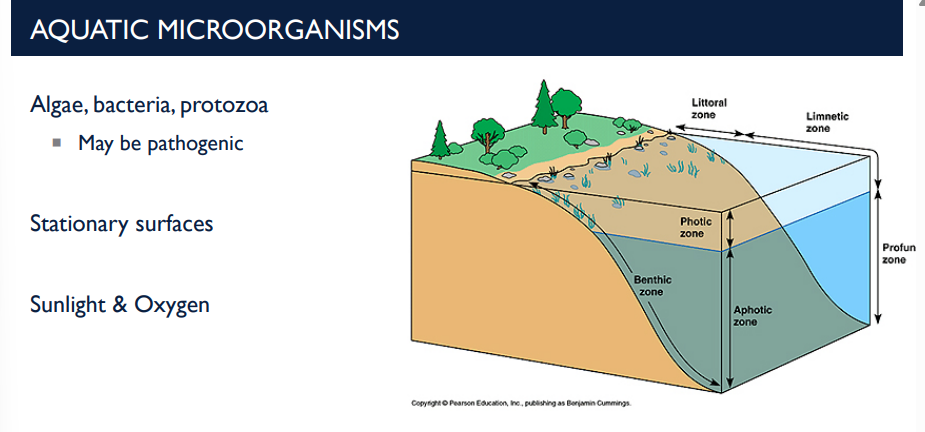
What are aquatic microorganisms and their characteristics?
These are microorganisms that live in water environments, including algae, bacteria, and protozoa.
Stationary surfaces: Microbes often attach to surfaces in water (rocks, pipes, plant surfaces).
They can form biofilms, which are colonies embedded in a protective matrix.
Sunlight & Oxygen: Sunlight drives photosynthesis in algae and cyanobacteria.
Oxygen is produced by photosynthetic microbes and used by aerobic bacteria.
Light and oxygen influence microbial growth and distribution in aquatic ecosystems.
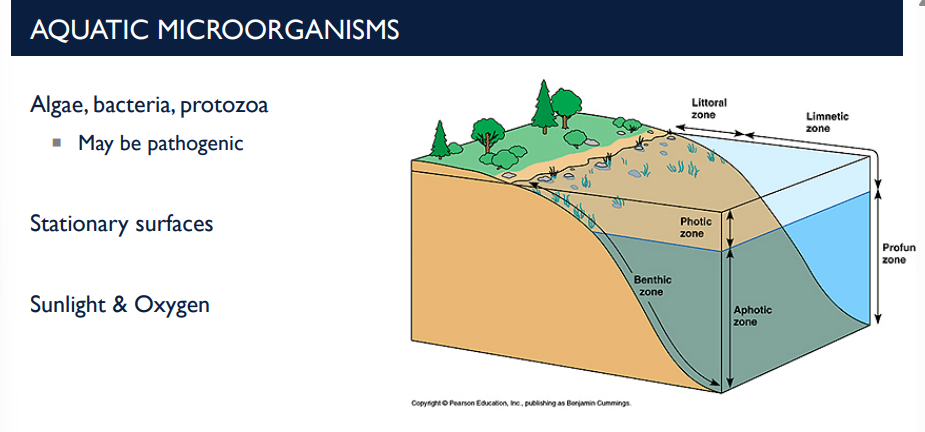
T/F: Algae, bacteria, & protozoa, different aquatic microorganisms, cannot be pathogenic.
F, they MAY be pathogenic
Water testing is used to check for fecal contamination and the possible presence of pathogens. What are indicator organisms? What is an example of one?
An indicator organism is a microbe that signals potential contamination without testing for every pathogen directly.
Present in human feces: Indicates possible contamination from sewage or waste.
Survive in water at least as well as the pathogens: If the indicator is present, pathogens might survive too.
Simple detection method: Easier and cheaper to test for than the pathogens themselves.
Example: Coliform bacteria. Presence suggests water may be unsafe to drink
Describe the characteristics of coliforms, a bacteria used as an indicator organism for water testing.
Nonpathogenic
Gram negative rods
Facultative anaerobes
Non-endospore-forming
Ferment lactose (acid and gas)
Produce β-galactosidase
Escherichia coli is a type of fecal ______ that produces beta-____
fecal coliform that produces B-glucuronidase
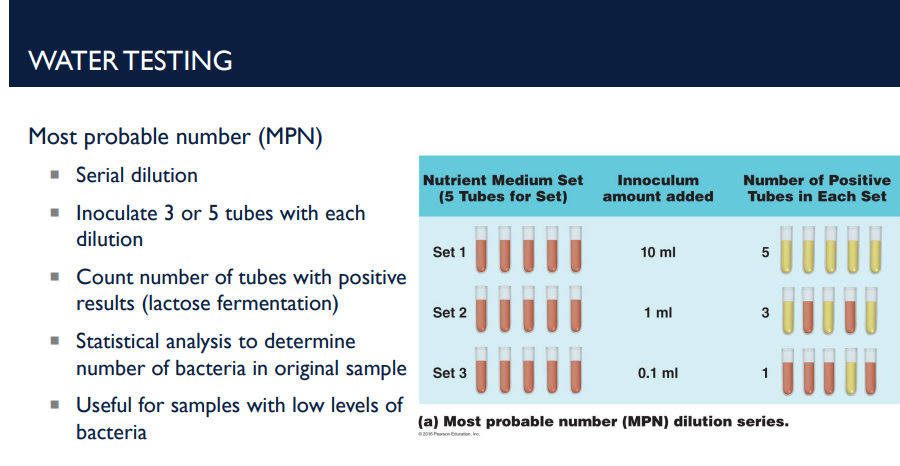
In the context of water testing, what is the MPN (most probable number)? No need to describe the process, just describe what it does
The Most Probable Number (MPN) method is a statistical way to estimate the number of bacteria in a water sample, especially when bacterial numbers are low.
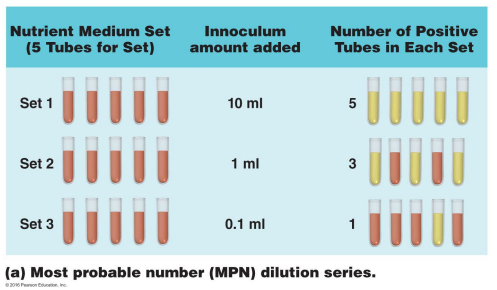
In the context of water testing, describe the process of most probable number (MPN)
Step-by-Step Process
Serial Dilution
The water sample is diluted step by step to reduce bacterial concentration.
Inoculate Tubes
3 or 5 tubes are inoculated for each dilution level.
Tubes contain lactose broth to test for lactose-fermenting bacteria (like coliforms).
Observe Positive Results
A positive tube shows lactose fermentation, usually indicated by a color change or gas production.
Count Positive Tubes
Record the number of positive tubes at each dilution.
Statistical Analysis
Use MPN tables to estimate the number of bacteria in the original sample based on the pattern of positives.
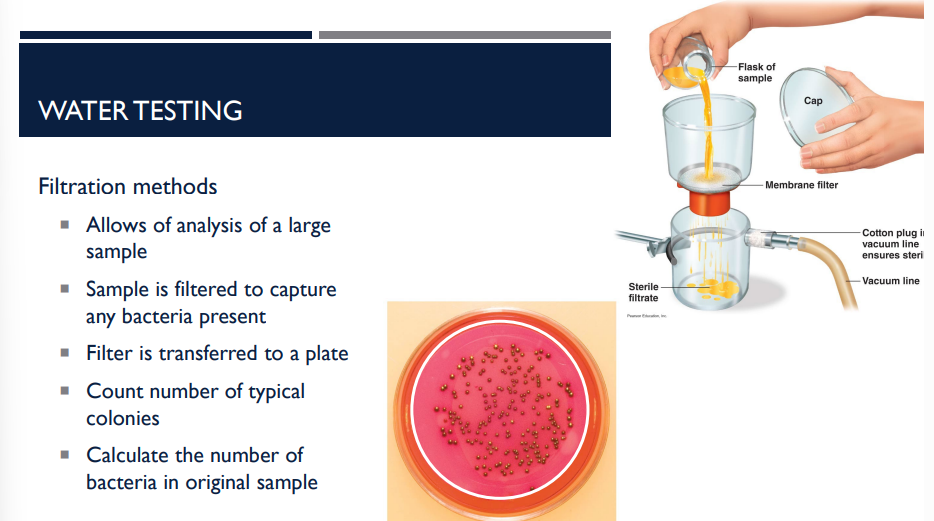
In the context of water testing, describe the process of filtriation methods.
Allows of analysis of a large sample
Sample is filtered to capture any bacteria present
Filter is transferred to a plate
Count number of typical colonies
Calculate the number of bacteria in original sample
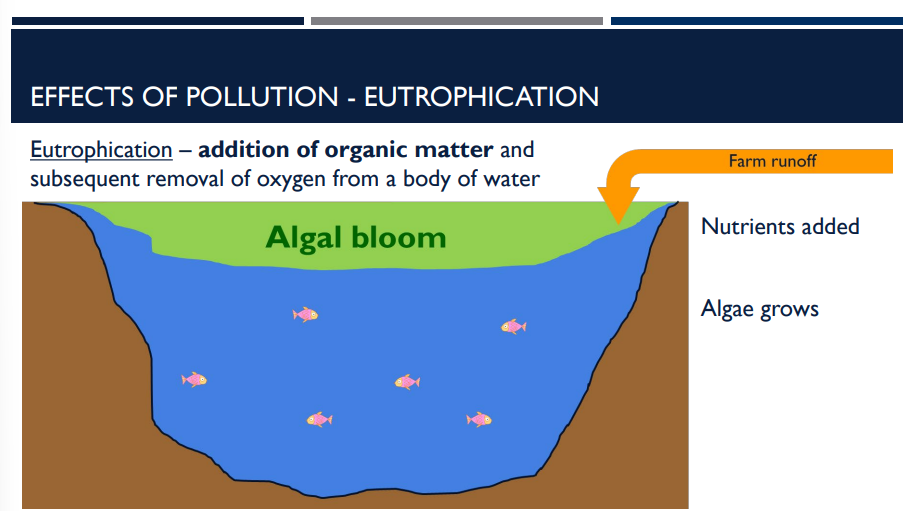
There are many effects of pollution, including Eutrophication. Describe Eutrophication.
the addition of organic matter and subsequent removal of oxygen from a body of water
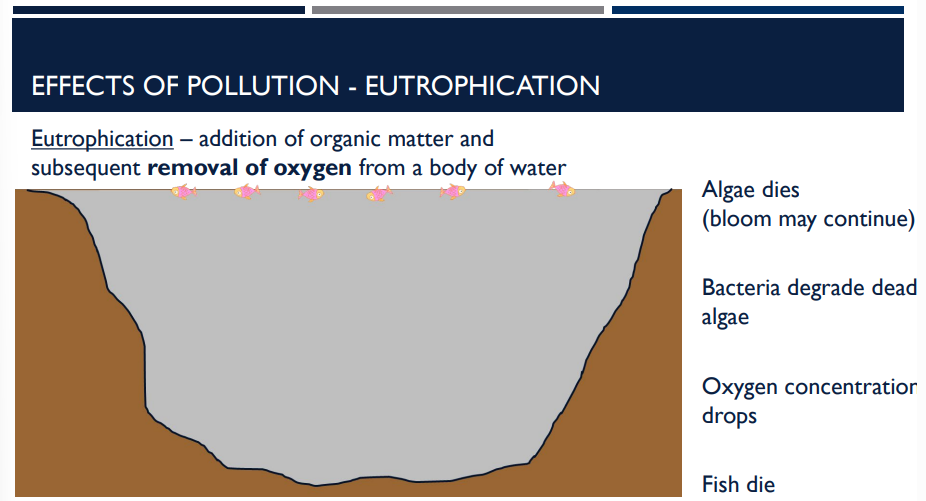
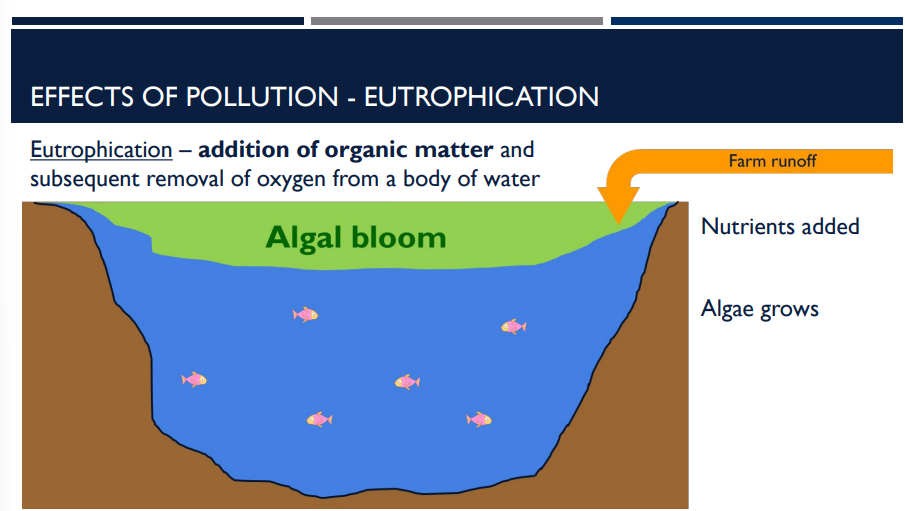
the addition of organic matter and subsequent removal of oxygen from a body of water is an effect of pollution called _____
Eutrophication
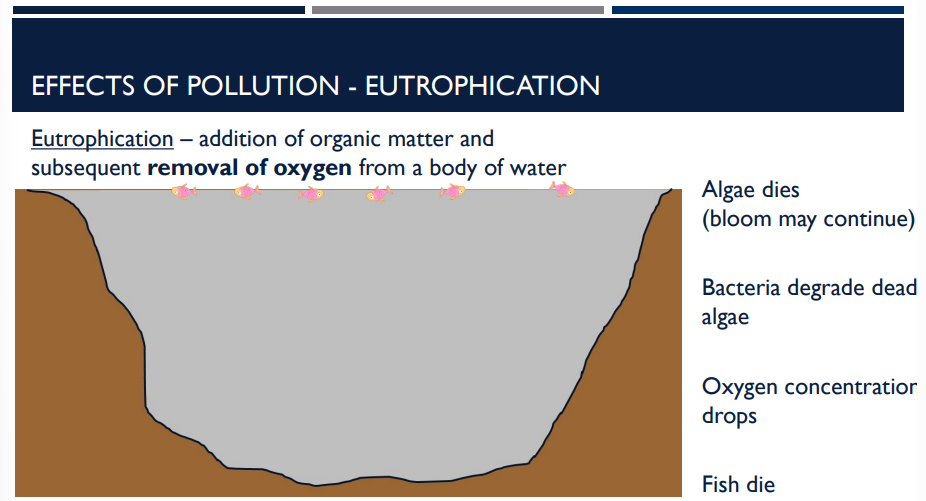

What effect of pollution is shown?
Eutrophication: the addition of organic matter and subsequent removal of oxygen from a body of water
There are many effects of pollution, which lead us to utilize bioremediation. Describe bioremediation.
Use of microbes to degrade pollutants
Oil spills
Drilling accidents

We can use microbes to degrade pollutants such as oil spills & drilling accidents. This is called
bioremediation: the use of microbes to degrade pollutants
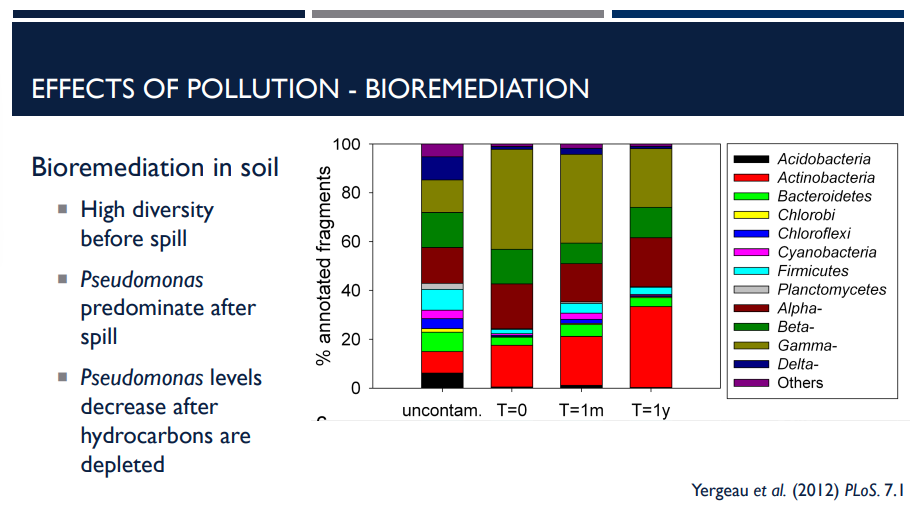
Bioremediation is the use of microbes to degrade pollutants like with oil spills. In soil, there must be a high diversity before the spill. What does that mean?
Natural soils usually contain a diverse microbial community.
Many types of bacteria, fungi, and other microbes coexist.
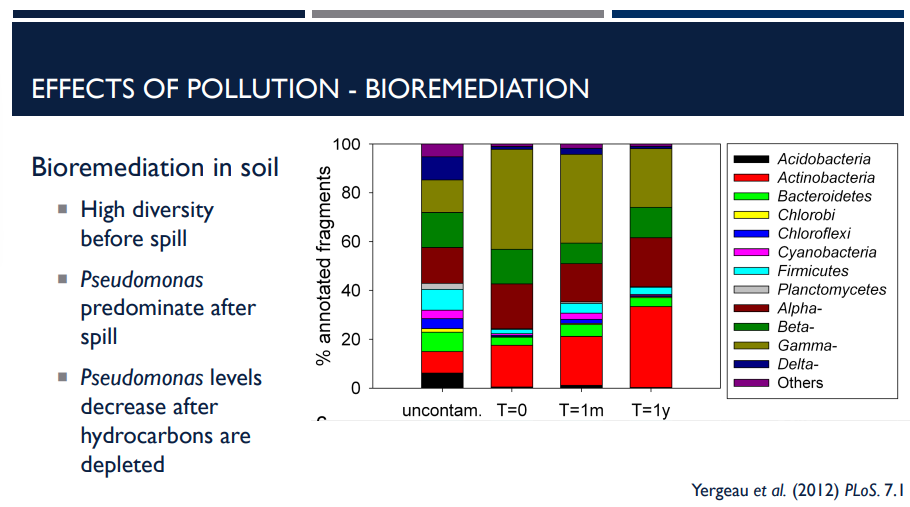
Bioremediation is the use of microbes to degrade pollutants like with oil spills. In soil, there must be a high diversity of of a microbial community before the spill. Which bacteria predominates after the spill?
Pseudomonas Predominate After Spill
When a hydrocarbon spill occurs (oil, fuel, etc.), the environment favors microbes that can use hydrocarbons as food.
Pseudomonas species often dominate because they can efficiently degrade hydrocarbons.
Pseudomonas levels decrease after hydrocarbons are depleted.
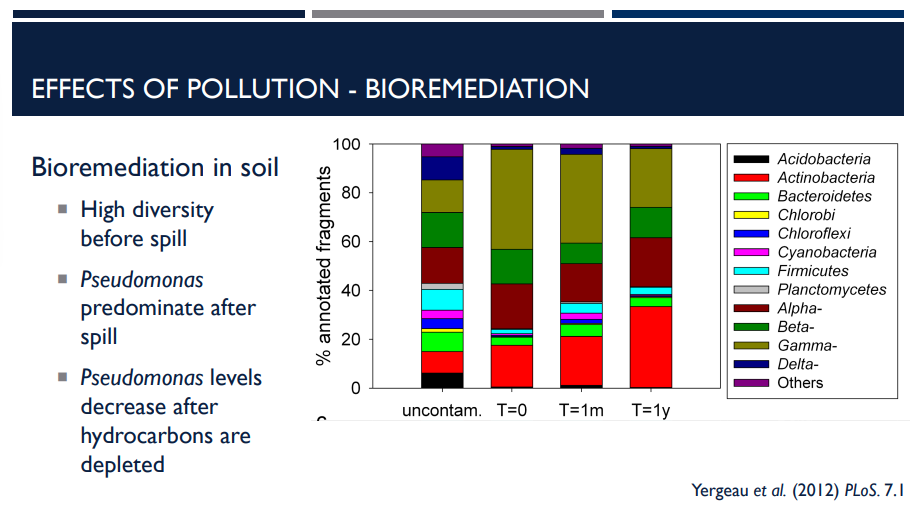
Bioremediation is the use of microbes to degrade pollutants, like with oil spills. In soil, there must be a high diversity of of a microbial community before the spill. Pseudomonas predominates after the spill. When do their levels decrease?
after hydrocarbons are depleted.