S&H Ch 104, 105, 107;Inflammation and Coagulation/Thrombosis MDRs;Cornell Coagulation and Platelet Videos
1/252
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
253 Terms
What is normal hemostasis a balance between?
Normal hemostasis is a balance between prothrombotic and antithrombotic tendencies
Antithrombotic usually predominates so blood can circulate as a liquid
Achieved by intact endothelium separating the cellular and plasmatic components of blood from the prothrombotic substances in the subendothelium
Endothelium also produces several antithrombotic substances with either antiplatelet (e.g. prostacyclin, nitric oxide) or anticoagulant (e.g. antithrombin, thrombomodulin) activity
If there is blood vessel damage there is a rapid switch from antithrombotic to prothrombotic
Clot formation traditionally considered two distinct processes, primary hemostasis and secondary hemostasis
Coagulation Cascade Diagram
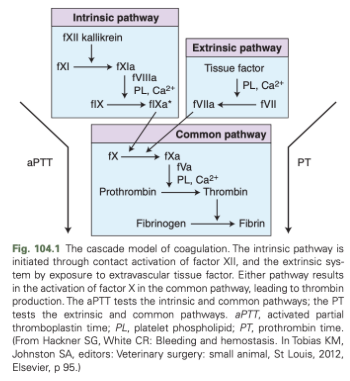
Components of Primary Hemostasis
Platelet number
Platelet function
vWF
Signs of Dysfunction of Primary Hemostasis
Small spontaneous superficial and mucosal bleeds
Excessive postoperative/postprocedural bleeding
Diagnostic Test Options for Primary Hemostasis
Platelet count
Platelet indices (MPV, PCV)
BMBT
Platelet closure time
Platelet aggregometry
Platelet procoagulant assays
Viscoelastic testing
vWF:Ag
Therapeutic Strategies for Primary Hemostasis Disorders
Treat primary underlying disease (e.g. immunosuppression)
Platelet containing transfusions
Desmopressin
RBC containing transfusions
± antifibrinolytic drugs
Components of Secondary Hemostasis
Clotting factors
Cofactors (e.g. calcium, vitamin K)
Signs of Dysfunction of Secondary Hemostasis
Large volume spontaneous bleeds
Excessive postoperative/postprocedural bleeding
Diagnostic Test Options for Secondary Hemostasis
PT
aPTT
ACT
Viscoelastic testing
Thrombin generation
Individual factor analysis
Therapeutic Strategies for Secondary Hemostasis Disorders
Treat primary disease
Clotting factor containing transfusion
Cofactor replacement (e.g vitamin K, calcium gluconate)
± antifibrinolytic drugs
Components of Fibrinolysis
Plasmin
tPA
Signs of Dysfunction of Fibrinolysis
Large volume spontaneous bleeds
Excessive postoperative/postprocedural bleeding
Diagnostic Test Options for Fibrinolysis
D-dimers
Viscoelastic testing
Therapeutic Strategies for Fibrinolysis Dysfunction
Antifibrinolytic drugs
Plasma-based transfusion
What are the three overlapping phases of the cell based model of coagulation?
Initiation
Amplification
Propagation
Cell Based Model of Coagulation Diagram
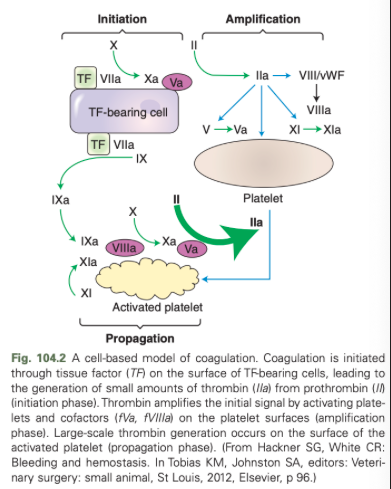
What are the initiating trigger for hemostasis in the cell based model of coagulation?
Tissue factor laden cells are the major initiating trigger for hemostasis
Analogous to the extrinsic pathway in secondary hemostasis
What occurs during the amplification phase of the cell based model of coagulation?
Small amount of thrombin made during the initiation phase which supports platelet activation during the amplification phase
What happens in the propagation phase of the cell based model of coagulation?
Most closely resembles the intrinsic and common pathways, supports massive thrombin generation
What are primary hemostatic disorders due to?
Reduced platelet number (thrombocytopenia) or abnormal platelet function (thrombopathia)
What are mechanisms of action of thrombocytopenia?
Decreased production
Increased destruction
Consumption
Sequestration
Non-pathological
What are differential diagnoses for thrombocytopenia due to decreased production?
Primary marrow disease
Infectious
Immune mediated
Neoplastic
Idiopathic
Toxins/drugs
Radiation
What are differential diagnoses for thrombocytopenia due to increased destruction?
Primary ITP
Secondary ITP
Septic focus
Vector-borne disease associated (e.g. ehrlichiosis, anaplasmosis, RMSF, dirofilariasis)
Neoplasia
Vaccination
Drug recation
What are differential diagnoses for thrombocytopenia due to consumption?
Acute hemorrhage
Sepsis
Disseminated intravascular coagulation
Microagniopathies/ vasculitis
What are differential diagnoses for thrombocytopenia due to sequestration?
Splenic torsion
Hypersplenism
Severe hypothermia
What are non-pathological causes of thrombocytopenia?
Inherited macrothrombocytopenia (e.g. CKCS)
What are causes of acquired intrinsic platelet dysfunction?
Platelet inhibitors
Uremia
Synthetic colloids
Myeloproliferative disease
Pit viper envenomation
Disseminated intravascular coagulation
Trauma-induced coagulopathy
What are secondary hemostatic disorders associated with?
Clotting factor deficiency or failure of their activation
What are differential diagnoses for acquired secondary hemostatic disorders?
Vitamin K deficiency
Vitamin K antagonism
Liver dysfunction
Citrate overdose
Severe hypothermia
Acidemia
Anticoagulants
Hemodilution
Disseminated intravascular coagulopathy
Trauma-induced coagulopathy
Angiostrongylus vasorum infection
What can disorders of accelerated fibrinolysis be associated with?
May occur associated with massive trauma (trauma-induced coagulopathy), acute liver dysfunction, and spontaneous hemoperitoneum
What are traditional hemostatic tests best suited for?
Detection of hypocoagulability
Buccal Mucosal Bleeding Time (BMBT)
Most common method of assessing platelet function in small animals
Normal BMBT is typically <3 minutes in dogs and <2 minutes in cats
Crude indicator of primary hemostasis
Extraneous factors such as hematocrit and blood viscosity also influence the speed of clot formation
Platelet Closure Time
Obtained with an automated benchtop platelet function analyzer and provides similar infiromation to BMBT
How can platelet function be assessed?
With aggregometry or flow cytometry
Whole blood multiple electrode impedance aggregometry is the option most conducive for clinical use
Prothrombin Time (PT)
Measured by adding tissue factor to a patient blood sample
Assesses the extrinsic and common pathways
Activated partial thromboplastin time (aPTT)
Assesses intrinsic and common pathways
Involves the addition of kaolin and a source of phospholipids to patient blood
Activated Clotting Time (ACT)
Modified aPTT with utility in situations where a high concentration of heparin is present (e.g. extracorporeal blood circuits used for hemodialysis)
PT and aPTT in Critically Ill Patients
Prolonged PT with normal aPTT - issue with extrinsic and/or common pathways (e.g. factor VIII deficiency in hemophilia A, synthetic colloid administration)
Common to see prolongation of both PT and aPTT in the critically ill Vitamin K-dependent factor deficiency (factors II, VII, IX, X)
Results in PT prolongation in excess of aPTT since factor VII has the shortest half-life of these factors
Mild to moderate prolongation of aPTT is commonly seen in patients with systemic inflammation but doesn't normally correspond to increased bleeding risk
Point-of-care aPTT results are more prone to inaccuracy than PT
If abnormalities in these screening tests are identified, consider measurement of individual clotting factors on a case by case basis
How to you evaluate fibrinolysis?
Fibrin(ogen) degradation products and D-dimer assays
Animals with DIC usually have elevated D-dimers
D-dimers are nonspecific indicators of clot turnover and should not be used as a sole indicator or DIC
What does viscoelastic monitoring provide information on?
The rate of clot formation, clot strength, and rate of fibrinolysis
What are viscoelastic variables that are indicators of rate of clot formation?
Reaction (R) time (TEG), Clot time (CT) (ROTEM), Clot time (CT) (VCM)
Kinetics (K) (TEG), Clot formation time (CFT) (ROTEM), Clot formation tests (CFT) (VCM)
Alpha (angle) (TEG), Alpha (ROTEM), Alpha (VCM)
What is reaction (R) time/clot time(CT)/clot time (CT) affected by?
Factors XII and XI
What is kinetics (K)/clot formation time (CFT)/clot formation tests (CFT) affected by?
Fibrinogen, factor XIII, and platelets
What is alpha (angle)/alpha/alpha affected by?
Fibrinogen, factor XIII, and platelets
What are viscoelastic indicators of clot strength?
Maximum amplitude (MA) (TEG), maximum clot firmness (MCF) (ROTEM), maximum clot firmness (MCF) (VCM)
Elastic shear modulus (G) (TEG)
What is maximum amplitude (MA)/maximum clot firmness (MCF)/maximum clot firmness (MCF) affected by?
Affected by platelets and fibrinogen
What does elastic shear modulus (G) indicate?
Indicates maximum clot strength
What is elastic shear modulus (G) affected by?
Platelets and fibrinogen
Elastic Shear Modulus (G) Equation
G = 5000 x MA/(100-MA)
What is a viscoelastic indicator of the rate of fibrinolysis?
Lysis (Ly/30/60) (TEG), Clot lysis (CL30/60) (ROTEM), Lysis index (Li30/45) (VCM)
What is lysis (Ly30/60)/clot lysis (Cl30/60)/lysis index (Li30/35) affected by?
Plasmin activity
What are monitoring options for aspirin?
PFA-100 (using collagen-epinephrine)
Platelet aggregometry (using arachidonic acid)
TEG-platelet mapping (using arachidonic acid)
What are monitoring options for clopidogrel?
PFA-100 (using ADP-collagen)
Platelet aggregometry (using ADP)
TEG-platelet mapping (using ADP
Monitoring Options for Warfarin
aPTT
INR
Monitoring Options for Unfractionated Heparin
Drug specific anti-Xa activity
PT
ACT
TF and kaolin activated TEG (RapidTEG)
Monitoring Options for Low Molecular Weight Heparin
Drug specific anti-Xa activity
Monitoring Options for Direct Oral Anticoagulants
Drug specific anti-Xa activity
PT
Platelet Counts in Thrombocytopenia
Degree of thrombocytopenia can be a helpful indicator of the likely cause of the disease
Consumptive processes such as acute hemorrhage, sepsis, and vasculitis typically result in platelet counts between 50,000 and 90,000/ul
Increased destruction, decreased production, ehrlichiosis infections, and DIC typically lead to more severe thrombocytopenia (<20,000/ul)
Platelet count alone is an imperfect means of assessing primary hemostatic competence
Spontaneous and procedural bleeding is unlikely when platelet counts are >50,000/ul provided no other hemostatic dysfunction is present
Acquired Platelet Dysfunction
May occur with NSAID toxicity
Dose dependent coagulopathy, characterized as an acquired vWF and factor VIIIa deficiency, described in animals administered large volumes of synthetic colloids
May be encountered in disease states such as uremia, pit viper envenomation, and trauma-induced coagulopathy
Acquired Clotting Factor Dysfunction
Acquired secondary hemostatic disorders are associated with a reduced number or activity of clotting factors
Hypoperfusion and massive transfusion of citrate phosphate dextrose adenine (CPDA)-containing blood products have been associated with severe acidemia that may impair clotting factor activity
Clotting factor consumption (e.g. DIC) is another mechanism of secondary hemostatic dysfunction
Calcium is an important cofactor for clotting factors
Acute hypocalcemia may occur associated with citrate toxicity (e.g. massive transfusion) and may contribute to a hypocoagulable tendency
Dilutional Coagulopathy
Animals with acute hemorrhage will lose whole blood and consume clotting factors and platelets in response to hemorrhage
Compensatory fluid shifts of clotting factor deficient fluid from the interstitium to the intravascular compartment may occur, in addition to the administration of synthetic replacement fluids devoid of clotting factors
Vitamin K Absence or Antagonism
Vitamin K absence or antagonism is the most common reason for decreased clotting factor activity in small animals
Ingestion of anticoagulant rodenticides containing warfarin or coumadin
Inhibit vitamin K epoxide reductase, an enzyme needed for vitamin K recycling
Hydroquinone (reduced vitamin K) must be present for activation of the vitamin K-dependent coagulation factors (II, VII, IX, and X)
Vitamin K absence can also be caused by severe liver dysfunction and intestinal malabsorption
Liver Dysfunction Causing Coagulopathy
Normal hemostatic function is related to liver function since clotting factors, endogenous anticoagulants (e.g. protein C) and fibrinolytic proteins are synthesized in the liver
Hemostatic dysfunction typically occurs in the face of more advanced liver disease
Caused by reduced factor synthesis, vitamin K deficiency, and acquired platelet dysfunction
Chronic hepatopathies are less likely to exhibit hyperfibrinolytic tendency, but hypocoagulability is common with more severe disease
Hypercoagulability has been identified in some animals with liver disease (e.g. congenital portosystemic shunts) and could lead to serious adverse effects (e.g. portal vein thrombosis)
Spontaneous hemorrhage is less common in animals with liver disease but there is considerable concern for bleeding if these animals undergo invasive procedures
Disseminated Intravascular Coagulation
A precipitating trigger (e.g. inflammation, sepsis, heat stroke) incites systemic activation of coagulation, resulting in massive thrombin generation and widespread clot formation
Two major detrimental effects of extensive thrombosis
Microthrombosis may lead to tissue hypoperfusion and resultant tissue dsyfunction
Massive thrombosis leads to clotting factor consumption and fibrinolysis activation leading to a bleeding tendency
Early in the disease course, hypercoagulability and DIC will be asymptomatic (nonovert) and may be missed by conventional laboratory diagnostic tests
As consumption continues, more pronounced bleeding tendency will be appreciated (overt DIC)
Bleeding characteristics may have features of both primary and secondary hemostatic dysfunction
Potential hemostatic abnormalities in DIC include thrombocytopenia, prolongation of PT and aPTT, hypofibrinogenemia, elevated D-dimers, and reduced antithrombin activity
Trauma-Induced Coagulopathy
In people, an early-onset endogenous hemostatic dysfunction, termed trauma-induced coagulopathy (TIC) occurs in severely injured patients with shock
Mechanisms
Increased generation of activated protein C by high concentrations of thrombin-thrombomodulin and endothelial damage favoring an anticoagulant and hyperfibrinolytic state
Limited evidence to suggest this occurs in traumatized dogs and cats
Good evidence that an iatrogenic resuscitation-associated coagulopathy occurs in both people and animals secondary to dilution with synthetic fluids, combined with the presence of acidosis, hypothermia, and hypocalcemia
Hyperfibrinolylsis
Identified in some disease state, especially trauma-induced coagulopathy (TIC) and acute liver disease
Dogs with more severe hemodynamic compromise, assessed by a higher plasma lactate concentration and greater volume of plasma administered, were more likely to demonstrate a hyperfibrinolytic tendency
What are the three categories of treatment options for coagulopathy?
Administration of transfusion products
Hemostatic agents
Therapies aimed at addressing the primary disease process
Administration of Transfusion Products for Coagulopathies
Product used depends on the nature of the hemostatic dysfunction (e.g. platelet replacement for primary hemostatic disorders, clotting factor replacement for secondary hemostatic disorders)
Hemostatic Agents for Coagulopathies
Desmopressin - facilitates vWF release from the endothelium
Lysine analog antifibrinolytic drugs (e.g. epsilon aminocaproic acid, tranexamic acid)
Not recommended in DIC since they may worsen clotting factor consumption due to clot persistence
What are the three pillars of patient blood management?
Detection and treatment of hemorrhage
Reduction in further blood loss
Harnessing patient specific physiologic reserves
How do primary hemostatic defects manifest?
Surface or mucosal bleeding
How do disorders of secondary hemostasis present?
Body cavity hemorrhage, hematomas, subcutaneous, intramuscular, or joint hemorrhage
Generalized Bleeding
Occurring in multiple parts of the body
Localized Bleeding
Confined to one particular area
Differentials for Hemorrhage
Disorders of primary or secondary hemostasis
Hyperfibrinolysis
Traumatic tissue damage
Intra- or postoperative complications
Rupture of mass lesions
DIC can result in uncontrolled severe bleeding, has a high mortality rate
Most common cause of DIC in the ICU is sepsis
Common Acquired Primary Causes of Bleeding in the ICU
Immune-mediated thrombocytopenia
Bone marrow disease
Uremia
Hyperviscosity syndrome
Antiplatelet medications (clopidogrel)
Nonsteroidal antiinflammatory medications
DIC
Splenic Sequestration
Common Acquired Secondary Causes of Bleeding in the ICU
Liver failure
Anticoagulant medications (direct thrombin inhibitors, factor X inhibitors, warfarin)
Anticoagulant rodenticides
Consumptive processes
Extracorporeal therapies
Dilutional
Hypothermia (<34*C)
Acidemia (pH<7.2)
Hypocalcemia (ionized Ca <1 mmol/L)
Vitamin K1 deficiency
Common Causes of Fibrinolysis in the ICU
Acute traumatic coagulopathy
Liver disease
Neoplasia
Breed predisposition (Greyhounds)
Common Vascular Causes of Bleeding in the ICU
Infectious diseases (e.g. sepsis, rickettsial diseases)
Cushing’s disease
Hyperviscosity. syndromes
Drug-induced
Neoplasia
Trauma
Surgical bleeding
Ruptured Mass
Diagnostic Approach for Patients Exhibiting Cutaneous and Mucosal Bleeding
Automated platelet count in conjunction with a blood smear is the first diagnostic test of choice to rule out a severe thrombocytopenia
If platelet count is normal, platelet function testing can be performed
Requires fresh platelets that are tested ideally within 2.5 hours of collection
May not identify the specific reason for platelet dysfunction
Buccal mucosal bleeding time could be considered as a simple bedside test of platelet function
Disadvantages
Operator dependent variability
Lack of ability to detect mild defects in platelet function
Unreliable prediction of surgical bleeding
Platelet mapping viscoelastic test (VET) assays can quantify the degree of platelet dysfunction due to clopidogrel or aspirin administration
Insensitive for the diagnosis of platelet dysfunction and a severe decrease in platelet count and function must be present to affect the VET
Diagnostic Approach for Patients with Bleeding Consistent with Dysfunction of Secondary Hemostasis
Coagulation testing, consisting of activated clotting time (ACT), prothrombin time (PT), activated partial thromboplastin time (PTT), D-dimers, and VETs, should be considered
What results of coagulation testing are reflective of coagulopathy?
Prolonged clotting times >1.5-2 times the patient baseline or reference value
What is one of the most reliable predictors of massive transfusions in human patients?
An international normalized ratio (PT standardized to thromboplastin reagent) >1.5
What are prolongations of PT and PTT associated with in human and veterinary patients?
Blood loss and increased mortality
Sensitivity of Coagulation Tests
Not much evidence that the standard plasma-based coagulation tests accurately reflect a clinical coagulopathy or are useful in guiding transfusion therapy in bleeding patients
Standard coagulation tests are an insensitive indicator of clotting factor deficiency with 60-70% of the factor level decrease necessary for prolongation of PT and PTT
ACT is less sensitive than PTT with less than 10% of factor activity necessary for the ACT prolongation
ACT, unlike PTT< may be affected by severe thrombocytopenia and may be increased in patients with platelet counts of less than 10,000/uL
VET is a more sensitive diagnostic modality for identifying hemostatic abnormalities in bleeding patients
Advantage is its ability to evaluate the interaction of cellular components and coagulation factors, which is more indicative of in vivo hemostasis
VET-guided transfusion protocols have been documented to result in less blood product use, decreased morbidity, and possibly decreased mortality than protocols based on standard tests of hemostasis
Imaging is useful for quantifying the degree and extent of the hemorrhage as well as identifying potential anatomic targets to address
What is the ideal resuscitative fluid for significant blood loss?
Whole fresh blood (WFB)
When is isotonic crystalloid therapy the recommended resuscitation fluid?
Isotonic crystalloid therapy is the recommended resuscitation fluid for the initial treatment of patients suffering from hemorrhagic shock, especially when blood product therapy is not immediately accessible
Recommendations for resuscitation have recently shifted, with current guidelines favoring the administration of conservative volumes of crystalloids to avoid dilution of blood cells and coagulation factors, hypothermia, and acidosis, and to prevent formed clot dislodgement
What can be used for low volume fluid resuscitation?
Hypertonic saline
May be a crystalloid of choice in polytrauma patients with suspected traumatic brain injury
Synthetic Colloids for Hemorrhagic Shock Resuscitation
Synthetic colloids have been used for hemorrhagic shock resuscitation but are not superior to crystalloids and can inhibit platelet function as well as induce a coagulopathy
Administration of Blood Products for Hemorrhagic Shock
Administration of blood products in a 1:1:1 unit ratio of packed red blood cells (pRBCs):fresh frozen plasma (FFP): platelet concentrates, as well as additional fibrinogen concentrates in cases of severe hypofibrinogenemia, has proven beneficial in bleeding human trauma patients
This protocol has resulted in the lowest mortality rate when compared to other ratios
No evidence for an ideal ratio in veterinary medicine
Fresh platelet concentrates are routinely not available so reasonable to use a combination of FFP and pRBCs
Consider high ratios of FFP to pRBCs on a volume basis in veterinary patients with active bleeding
Target Hematocrit/Hemoglobin
Target hematocrit/hemoglobin concentration is controversial
Some recommend a higher hemoglobin threshold of 10 g/dl as a goal in actively bleeding humans and patients with comorbidities that may benefit from higher oxygen content
Conservative transfusion triggers that use a hemoglobin of 7 g/dl have been shown to be superior in some groups of bleeding patients
Hypotensive Resuscitation
Strategy to minimize further blood loss and hemodilution from nonsanginous fluid administration
Refers to maintaining mean arterial pressure (MAP) in the range of 60 mm Hg for up to 90 minutes
Typically culminates in the definitive management of hemostasis with surgery
TBI is a contraindication, must reestablish a MAP of 90 mmHg to preserve cerebral perfusion
Triad of Death
Acidemia + Hypothermia + Coagulopathy
How is the triad of death prevebted in bleeding ICU patients?
Prevention achieved with special focus on active rewarming and refraining from the administration of large quantities of crystalloids which results in dilutional anemia and coagulopathy
Correction of the underlying cause of acidemia should be attempted
Bicarbonate administration to correct acidemia has not been shown to improve outcome in acidemic, critically ill patients
Vitamin K for Control of Bleeding
Intake may be inadequate and its absorption and recycling may be compromised in patients with intrahepatic and posthepatic cholestasis
Administration at 0.5-1 mg/kg subcutaneously every 24 hours should be considered for coagulopathy associated with liver disease and biliary obstruction
Desmopressin (DDAVP) for Control of Bleeding
Synthetic analog of L-arginine-vasopressin
Most commonly used in bleeding patients to release von Willebrand factor and factor VIII from Weibel-Palade bodies in endothelial cells
Enhances the density of platelet surface glycoprotein receptors, increasing their adhesion potential
Dose of 1 mcg/kg subcutaneously should be considered in patients with evidence of platelet dysfunction
Could be repeated every 24 hours for up to 3 days, but there is about a 30% reduction in efficacy compared to initial administration
Antifibrinolytic Drugs for Control of Bleeding
Currently the mainstays of treatment of patients with severe bleeding due to acute traumatic coagulopathy, liver disease, and surgical bleeding associated with a number of procedures
Aminocaproic acid and tranexamic acid
Act by competitively inhibiting the lysine-binding sites on plasminogen, which prevents the conversion of plasminogen to plasmin in addition to direct inhibition of plasmin action
Higher doses might be needed in veterinary patients than in humans
In patients with severe traumatic hemorrhage, should be administered as soon as possible and preferably within 3 hours of the traumatic event
Structure of Hemoglobin
Hemoglobin is composed of four polypeptide chains (globins) each attached to a heme molecule
Heme is made up of a tetrapyrrole with a central iron molecule
Oxygen binds to the central iron molecule in the ferrous (Fe++) form
Each hemoglobin molecule carries four oxygen molecules
Pathophysiology of Carbon Monoxide Toxicity
CO is absorbed rapidly through the lungs at the level of the alveolus
Quantity of gas absorbed is dependent on minute ventilation (respiratory rate x tidal volume), duration of exposure, and concentrations of CO and oxygen in the environment
Once absorbed in the blood and circulated throughout the body, a small amount of CO is oxidized to CO2, some remains as gas in solution, and some binds to proteins including Hb, myoglobin, and cytochromes in mitochondria
What are the two main mechanisms of CO toxicity?
Impaired oxygen delivery to tissues (hypoxia via dyshemoglobinemia)
Direct cellular toxicity