Lewis Model (+electronegativity)
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
Why is the Lewis Model useful?
Shows how outer electrons are shared to fulfill the OCTET RULE
Explains structure of molecules
Introduced concept of BOND ORDER (single bond = bond order 1, double bond = bond order 2)
What is the Octet Rule?
The tendency of atoms to achieve the electronic configuration of the closest noble gas
What are the disadvantages to the Lewis Model?
Does not explain bonding
Model does not work for some molecules (eg, NO)
Fails to explain bonding in transition metal compounds
What is electronegativity?
The power of an electron to attract the electrons towards itself
What is Pauling’s electronegativity?
A scale that quantifies an element’s electronegativity
What equations did Pauling come up with, and how did he come to this conclusion?
Pauling noticed that XY (HETERONUCLEAR MOLECULES) bond dissociation enthalpies were greater than XX or YY (HOMONUCLEAR MOLECULES) bond dissociation enthalpies
XY > XX/YY
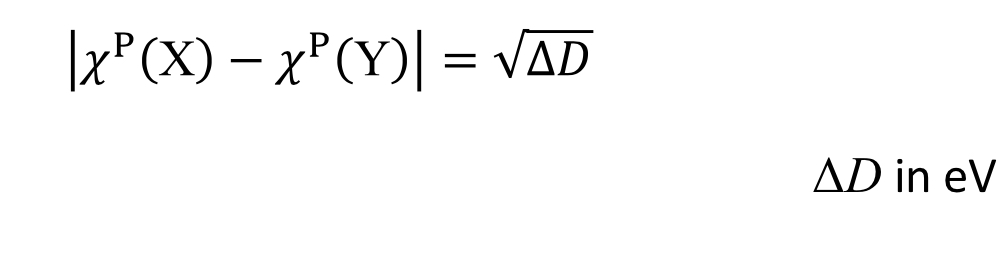
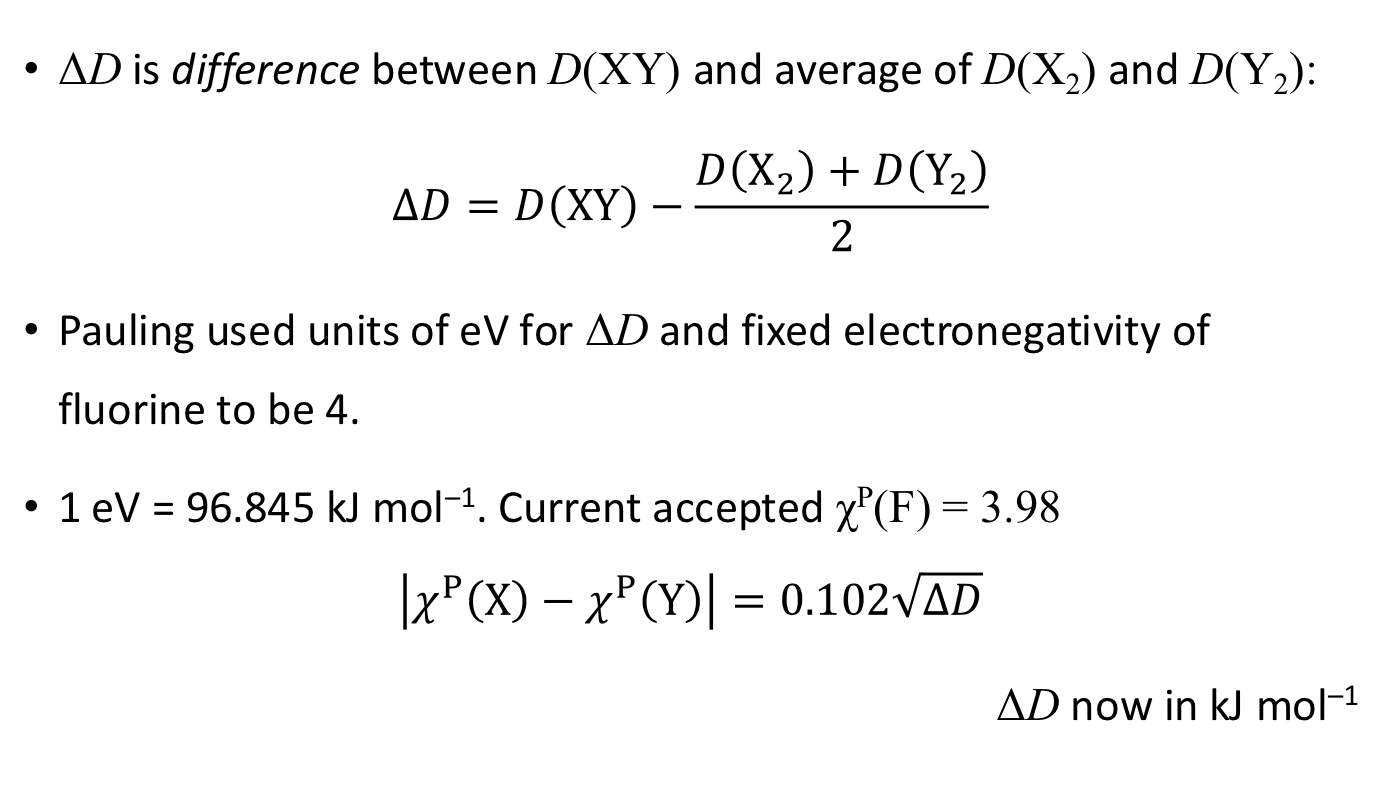
What other equation did Pauling derive from this?
What trends are found in electronegativity?
Electronegativity generally…
increases across period
decreases down group
How does oxidation state affect electronegativity?
Minor increases with increasing oxidation states