Year 7 - Particles
4.0(1)
4.0(1)
Card Sorting
1/38
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
39 Terms
1
New cards
gas
Moves randomly, particles don't touch and stay in the shape of its container
2
New cards
liquid
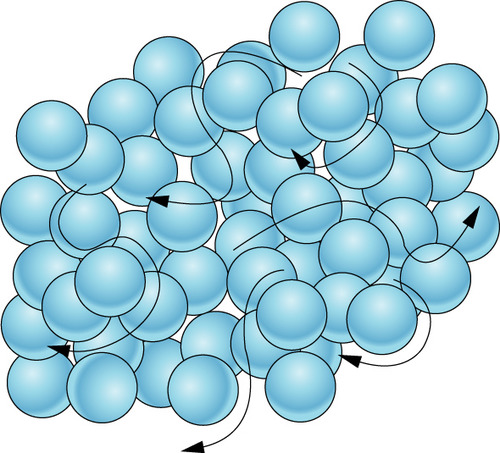
Moves randomly but still touches sometimes, stays in the shape of its container
3
New cards
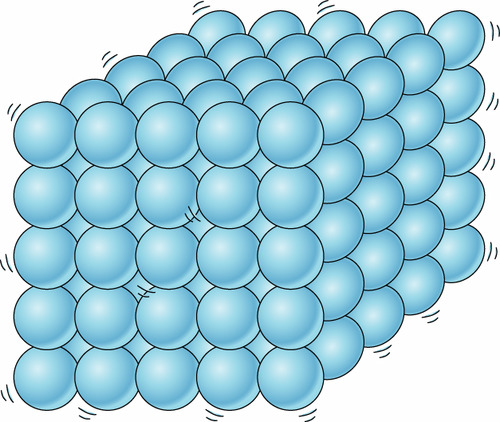
solid
stays in a fixed position, vibrates in the same position, are touching, does not stay in the shape of its container
4
New cards
solid, liquid, gas
Sort the three states of matter into order of increasing energy
5
New cards
gas, liquid, solid
Sort the three states of matter into order of decreasing energy
6
New cards
physical changes
What term can be used to describe interconversions between states of matter?
7
New cards
melting
What is the name for the physical change when a solid turns into a liquid?
8
New cards
freezing
What is the name for the physical change when a liquid turns into a solid?
9
New cards
boiling / evaporating
What is the name for the physical change when a liquid turns into a gas?
10
New cards
condensing
What is the name for the physical change when a gas turns into a liquid?
11
New cards
something that is made up of only one element or compound
What is a pure substance?
12
New cards
What is a mixture?
when there are two or more elements or compounds not chemically bonded
13
New cards
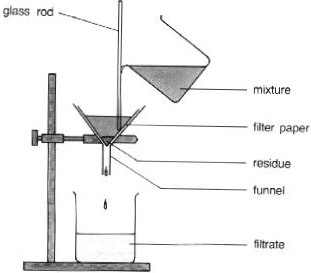
What process is this a diagram of?
filtration
14
New cards
Give the term being defined: The temperature at which a liquid boils.
boiling point
15
New cards
Give the term being defined: A specific temperature at which a solid turn into a liquid.
melting point
16
New cards
Give the term being defined: Solution passing through a filter.
filtrate
17
New cards
Give the term being defined: Describes a substance that cannot be dissolved in a certain liquid.
insoluble
18
New cards
Give the term being defined: Contains the maximum amount of solute that can dissolve in that amount of solvent at that temperature.
saturated solution
19
New cards
Give the term being defined: Substance that dissolves in a liquid to make a solution.
solute
20
New cards
Give the term being defined: Formed when a substance has dissolved in a liquid.
solution
21
New cards
Give the term being defined: The liquid in which a solute dissolves to make a solution.
solvent
22
New cards
Which state of matter takes a fixed position?
solids
23
New cards
Will substances expand when they are hotter or colder?
hotter
24
New cards
True or False: substances contract when they are colder
True
25
New cards
Saturated Solution
a mixture cannot dissolve any more solute because they do not have enough spaces between the particles
26
New cards
Pure substances
something that only contains one type of particle (can be chemically joined)
27
New cards
A mixture/ impure substance
2 or more substances that are mixed together but are not chemically joined.
28
New cards
Solution
A mixture that is made when a solid dissolves and mixes with a liquid
29
New cards
Filtering
Method used to separate an insoluble solid from a liquid
30
New cards
Insoluble
Something that is unable of dissolving
31
New cards
Soluble
Something that can be dissolved
32
New cards
Evaporation
Method used to separate a soluble solid from a liquid, also known as the process of turning a liquid to a gas
33
New cards
Dissolve
A solid that incorporates into a liquid to form a solution
34
New cards
Solute
A solid that dissolves
35
New cards
Solvent
A liquid that does the dissolving
36
New cards
Diffusion
movement of particles that allows them to spread out and mix with other particles, when particles move from an area of high concentration to an area of low concentration.
37
New cards
How does the kinetic energy of the particles increase?
it rises along with the temperature
38
New cards
All particles are attracted to each other: explain in depth
Some particles are attracted to each other strongly, but others attract to each other weakly
39
New cards
True or False: Not all substances are made of particles
False