Reactions of Alkenes
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
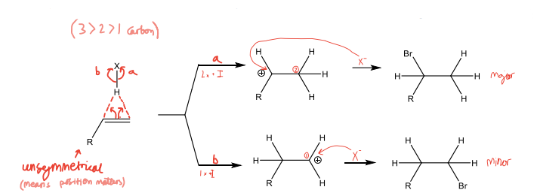
What is the Markovnikov rule
Markovnikov rule, On addition of HX to an unsymmetrical alkene in polar conditions, H attaches to the carbon with the fewest alkyl groups (least substituted or the carbon attached to the greatest number of H’s) and X to the carbon with the most alkyl groups.
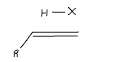
Draw the mechanism for this reaction in polar conditions, state whether is Markovnikov or anti Markovnikov
Markovnikov
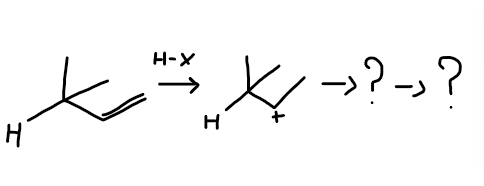
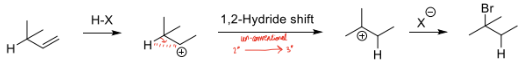
Complete the mechanism displaying how a hydride shift and explain why it does so, state whether is Markovnikov or anti Markovnikov
Undergoes a hydride shift to move electrons to a position of greater stability forming a more stable product.
Markovnikov
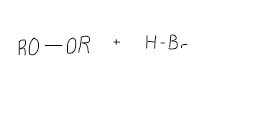
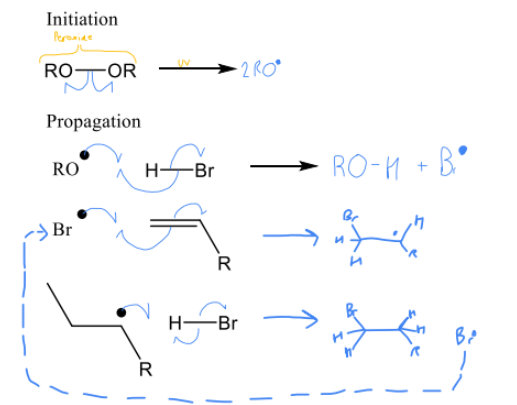
Complete the mechanism for addition of H-X in peroxides, state whether is Markovnikov or anti Markovnikov
anti Markovinikov
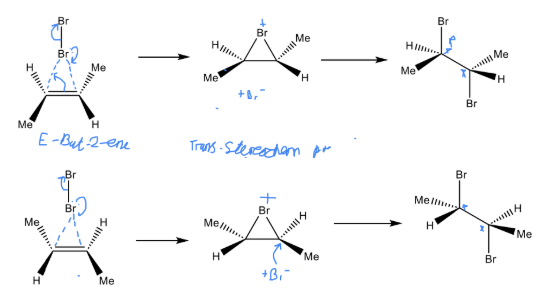
Describe the how stereochemistry effects the Addition of X
It will alter the stereochemistry of the products giving different mixtures of product depending on the configuration
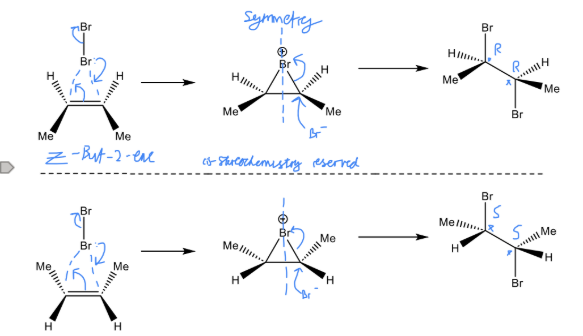
Describe the reaction of E butene with Br2 whilst also drawing the mechanism for it
Will undergo Markovinikov addition
Despite having two chiral centres they are optically inactive due to cancelling each other out (internal compensation)
They are neso compounds
50/50 likelihood of each reaction
Intermediates are a pair of enantiomers
Describe the reaction of Z butene with Br2 whilst also drawing the mechanism for it
Markovnikov addition
Forms a 1:1 mixture of enantiomers
Optically inactive
Racemic mixture forms
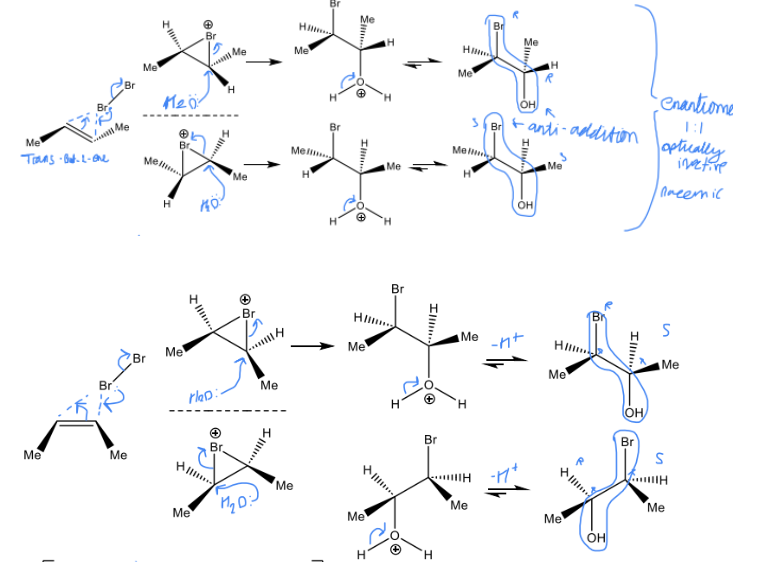
Draw the 4 reactions forming Bromohydrin from Enantiomers of But-2-ene reacted with Br2 and H2O
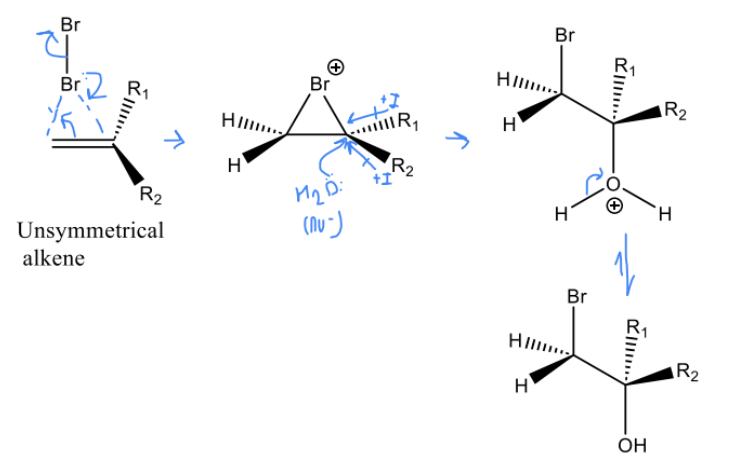
Draw the mechanism for Unsymmetrical alkene bromohydrin formation
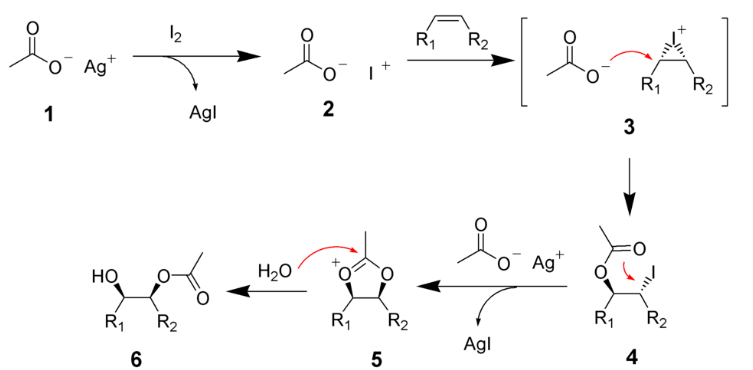
Draw the mechanism of Hydroxylation on an alkene, comment on the nature of the reaction
Not the best method to introduced OH due to being reversible, thus we need to force conditions.
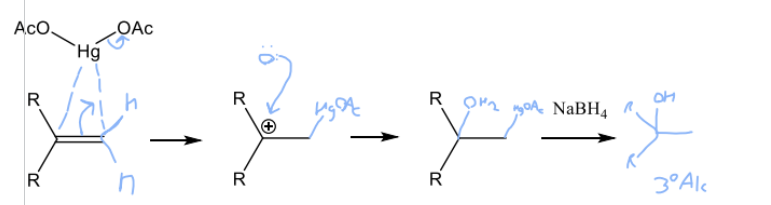
Draw the mechanism for oxymercuration on an alkene
Draw the Step 1 mechanism for hydroboration , and state whether it is Markovnikov or Anti-Markovnikov addition
Anti- Markovnikov
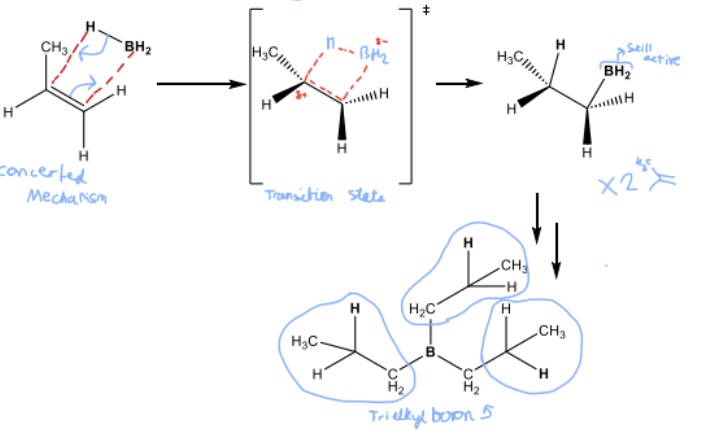
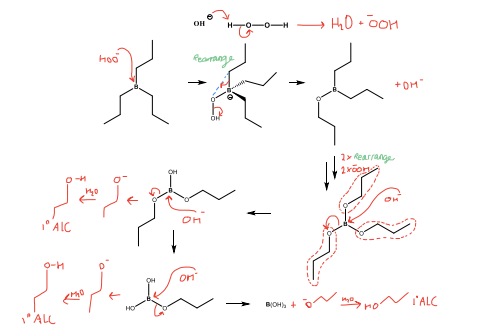
Draw the Step 2 mechanism for hydroboration, comment on number of moles reacted and produced
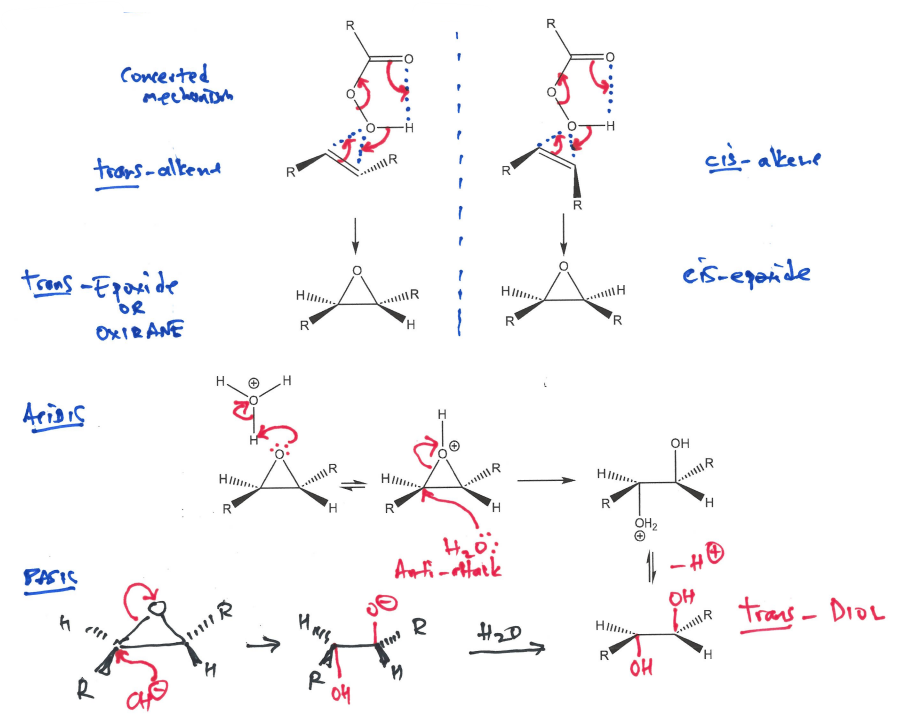
Draw out both mechanisms for epoxidation with carboxylic acid of a pair of enantiomers, do they give cis or trans products
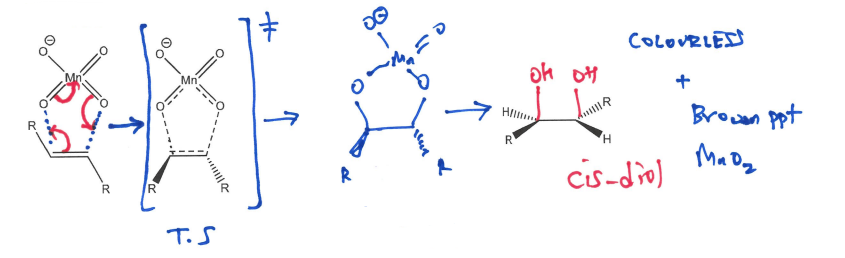
Draw out the Dihydroxylation of an alkene by reacting with MnO4, What’s this reaction called , does it give cis or trans products
Baeyer reaction
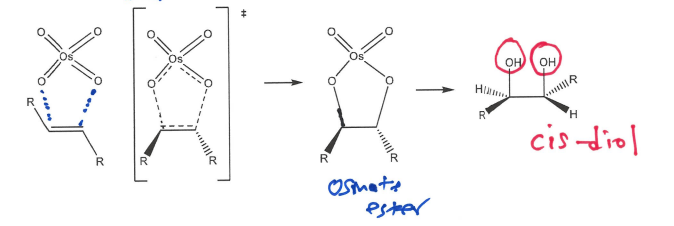
Draw out Dihydroxylation of an alkene by reacting with OsO4, does it give cis or trans products
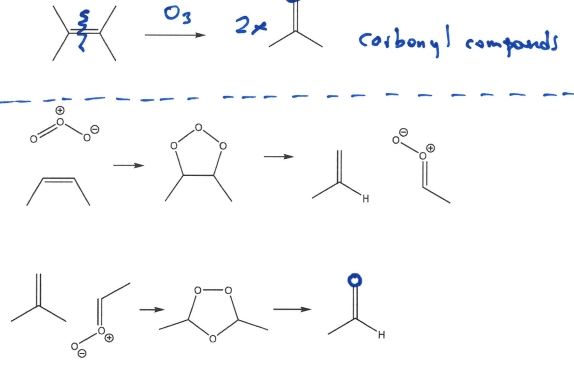
Draw out mechanism of Ozonolysis by reacting Alkenes with Ozone
Draw the mechanism for forming both cis and trans alkenes from the reduction of alkynes

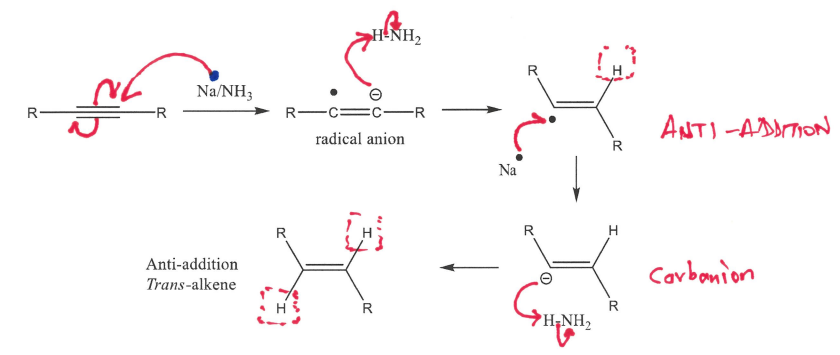
Draw the mechanism for forming a trans alkene from an alkyne in more detail, describe the type of addition
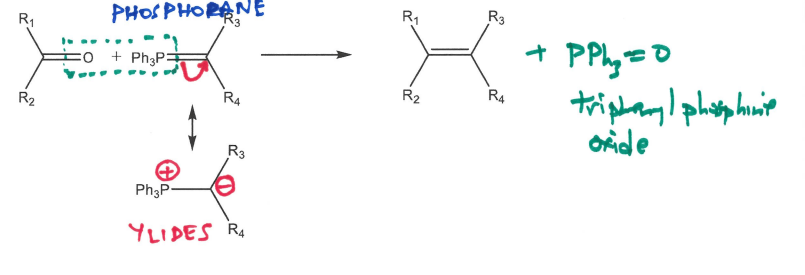
Draw the mechanism for the Wittig Reaction noting the names of important reactants