Chap 3: Enzymes
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
Q: What are enzymes and their key features?
A: Enzymes are globular proteins that catalyse metabolic reactions. They are biological catalysts
Q: How do intracellular and extracellular enzymes differ
A:Intracellular: made and used inside the cell. Extracellular: made in the cell but secreted to function outside.
Q: What does the lock-and-key model state?
A: The active site has a specific shape into which the substrate fits exactly.
Q: What does the induced fit model explain?
A: The substrate is partially complementary; the active site alters shape slightly for a better fit
Q: How do enzymes reduce activation energy?
A: By positioning substrates so bonds break more easily or by altering shape (induced fit) to facilitate conversion to products.
Q: Why is the enzyme reaction rate highest at the beginning?
A: Substrate concentration is high
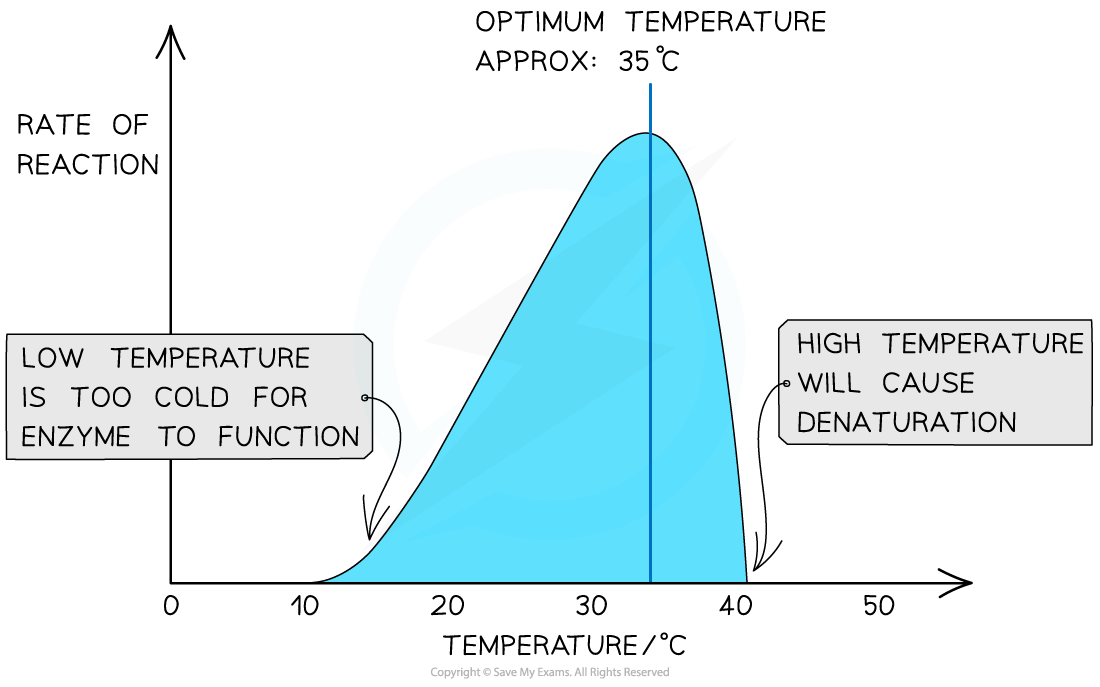
Q: How does temperature affect enzyme activity?
A:Low temperature → slow molecule movement. Increasing temperature → more collisions, faster reactions. Too high → enzyme denatures (H-bonds/ionic bonds break).
Optimum for humans ≈ 40°C.
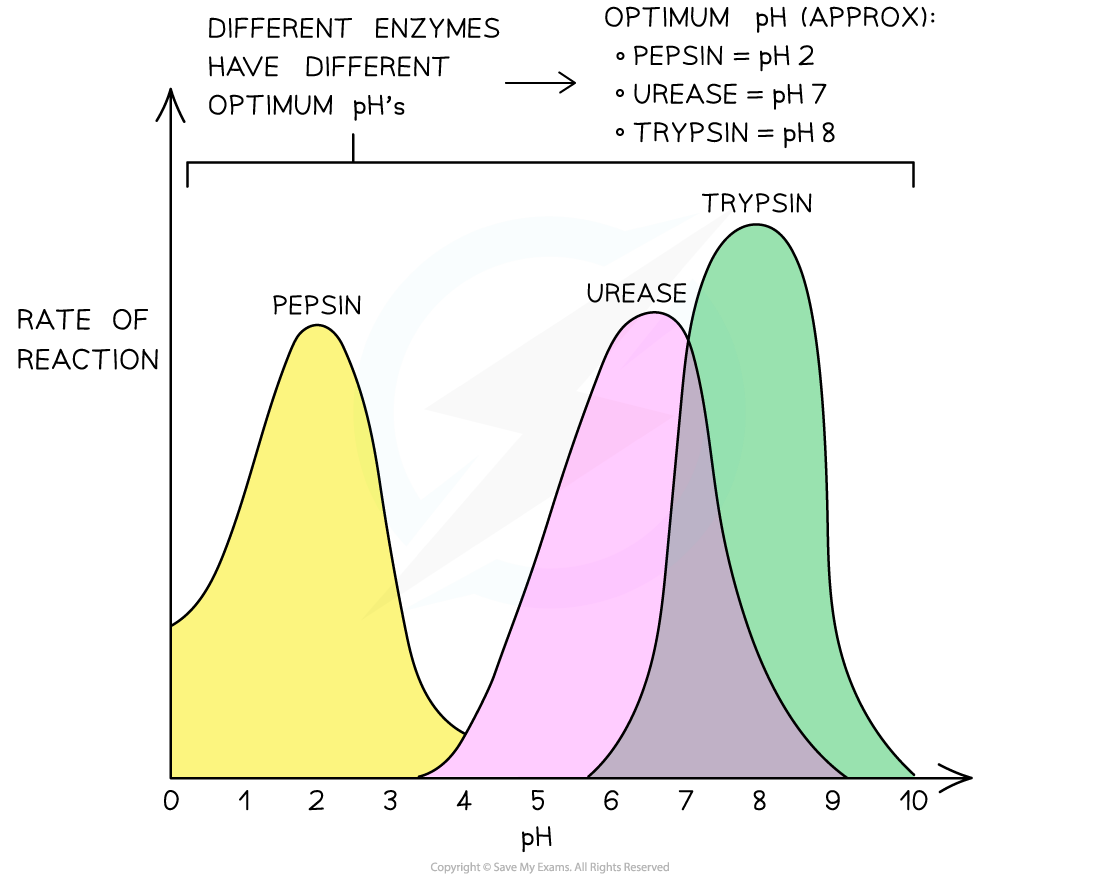
Q: How does pH change enzyme activity?
H⁺ ions affect R-groups and ionic bonds
Break the structure of active sites
Q: What happens as enzyme concentration increases?
A: Reaction rate increases linearly as long as substrate is abundant
Q: How does substrate concentration affect enzyme activity?
A: Rate increases with substrate concentration until Vmax
Q: What is competitive inhibition?
A: A molecule similar to the substrate competes for the active site. Can be reversed by increasing substrate concentration.
Q: What is non-competitive inhibition?
A: Inhibitor binds to an allosteric site
Q: What is end-product inhibition?
A: Final product of a pathway binds to the enzyme elsewhere
Q: What does Km represent?
A: Km is the substrate concentration at half Vmax. Lower Km = higher enzyme affinity for the substrate.
Q: How are enzymes immobilised using alginate beads
A: Enzyme mixed with sodium alginate → droplets added to calcium chloride → beads form
Q: What are advantages of immobilised enzymes?
A: Longer shelf life, Reusable, Easily recovered, Product not contaminated, Reduced product inhibition, Greater stability / less denaturation
Vmax
max rate of reaction at
saturating substrate conc
all of the active sites are occupied
inhibitor affects on the rate of reaction
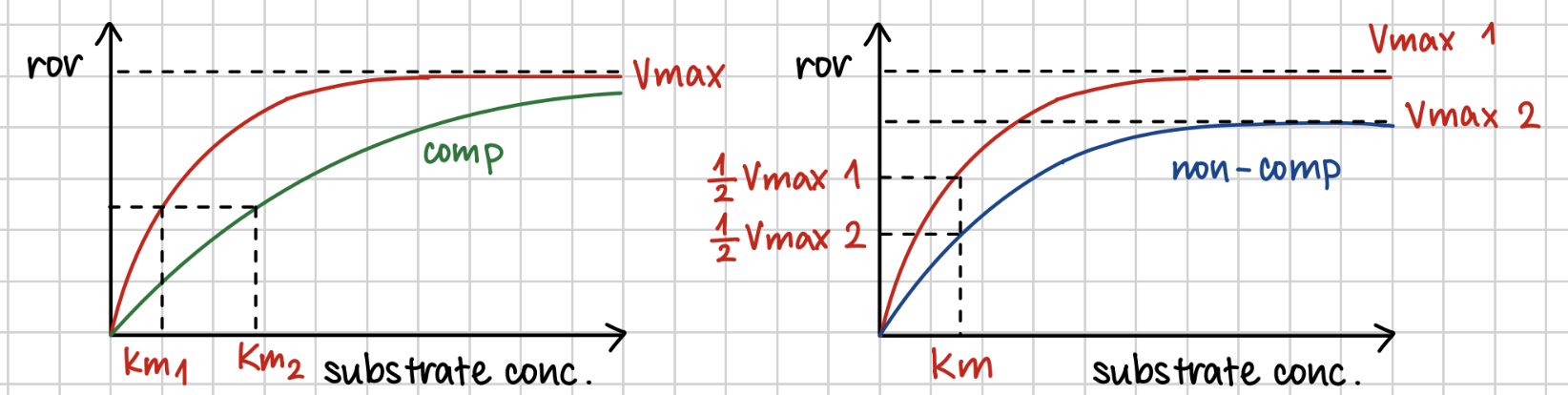
competitive inhi
→ Vmax unchanged
→ Km increase
non-competitive inhi
→ Vmax decreases
→ Km unchanged
affinity
enzymes willingness to bind to a substrate
enzymes affinity and Km correlation
Enzymes with lower value of km has a high affinity to its substrate and a higher value of km has a lower affinity.
increase substrate conc can…
increase rate of reaction when there are competitive inhi
no change when there are non competitive inhi