Electrophilic addition
1/5
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
6 Terms
What is an electrophile
These are electron acceptors and they are attracted to areas of high electron density
What are common examples of electrophiles
HBr
Chlorine and bromine halogens
Sulphuric acid
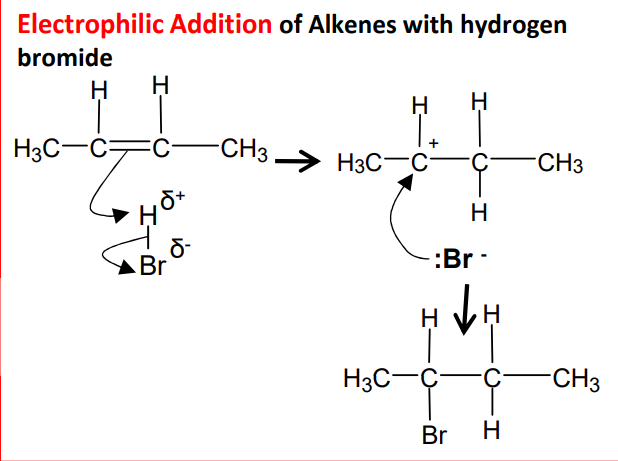
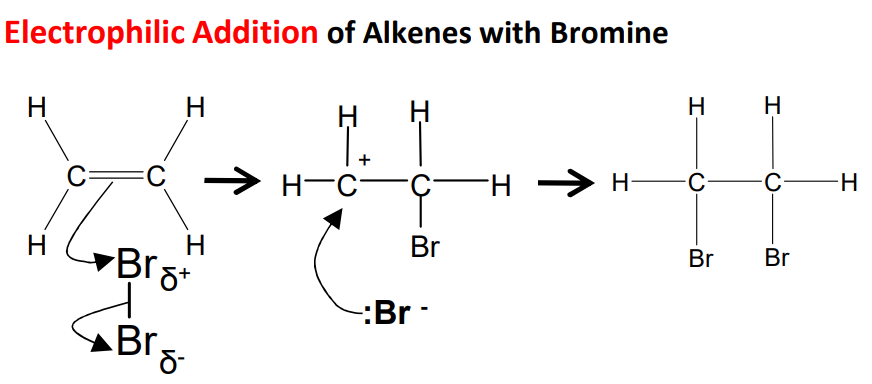
The mechanism for addition of halogens to alkenes, using bromine and ethene as an example
Step 1 - The high electron density of the C=C double bond repels electrons in the bromine molecule, polarising the Br-Br bond.
Step 2 - This leads to heterolytic fission of the Br-Br bond, with one bromine atom taking the bonding pair of electrons, creating a positively charged bromine which bonds to one of the carbon atoms.
Step 3 - A positively charged carbocation intermediate forms. The negatively charged bromide ion then bonds to the other carbon atom.
Step 4 - The final product, 1,2-dibromoethane, has a bromine atom bonded to each of the original alkene carbon atoms.
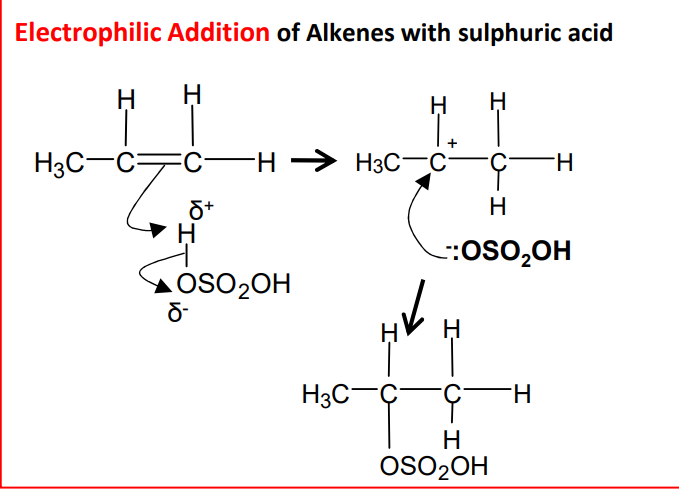
Electrophilic addition using sulphuric acid
Electrophilic addition using HBr