1.3 electrons, energy levels and atomic orbitals
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
13 Terms
Define shell
Energy level where electrons reside, representing regions of space at different distances from the nucleus
Define sub shell
Regions of the principle quantum shells where electrons exist in defined areas associated with particular amounts of energy.
Define orbital
Region around the nucleus where there is a high probability of finding an electron
Define principal quantum number
Number assigned to each energy level
Define ground state
The most stable, lowest energy configuration of an atom or molecule, where all electrons occur lowest possible energy levels/orbitals.
How many orbitals does the s sub shell have?
How many electrons does it hold?
1
2
How many orbitals does the p sub shell have?
How many electrons can it hold?
3
6
How many orbitals does the d sub shell have?
How many electrons can it hold?
5
10
Describe the order of increasing energy of sub shells
1s,2s,2p,3s,3p,4s,3d,4p,5s
How do electrons fill the orbitals?
They fill lower energy levels first and spread out in degenerate orbitals before pairing up with opposite spins to reduce electron-electron repulsion
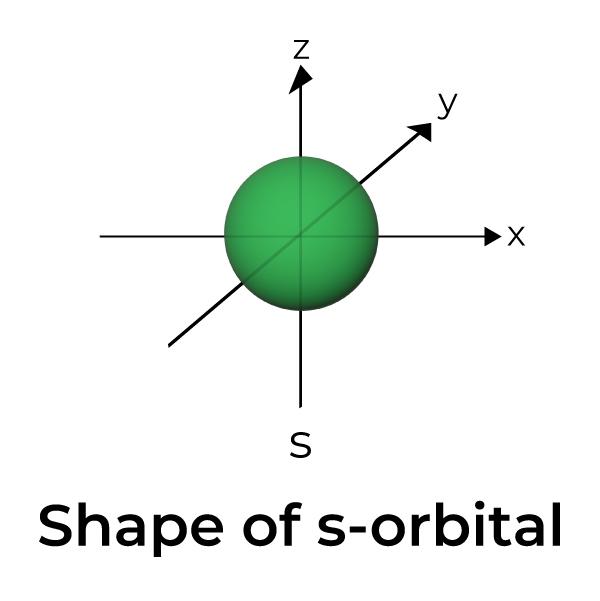
Draw the shape of an s orbital
Draw the shape of a p orbital
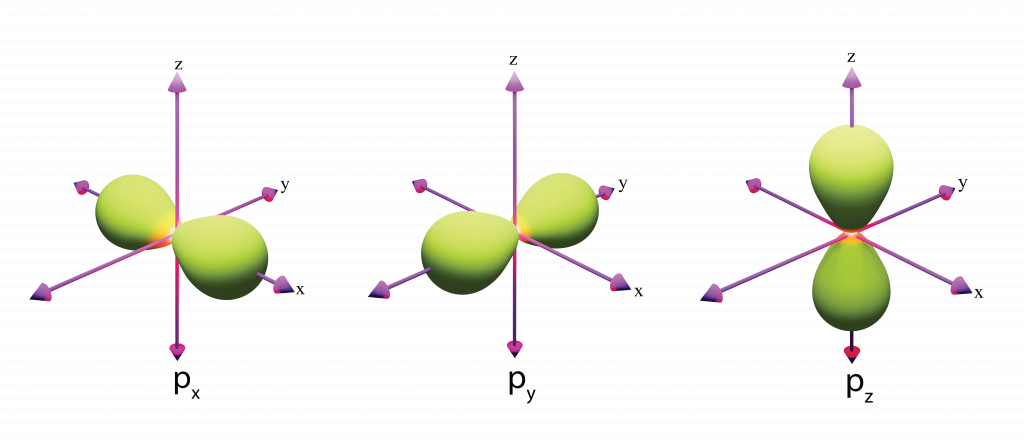
Define free radical
Species with one or more unpaired electrons