9-Free Radical Halogenations and Diels-Alder Reactions
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
because alkanes are unreactive since they have strong sigma bonds and atoms with no partial charges, what can you do to get them to react?
Cl2 or Br2, when light (hv), heat (∆), or peroxide (ROOR) are added
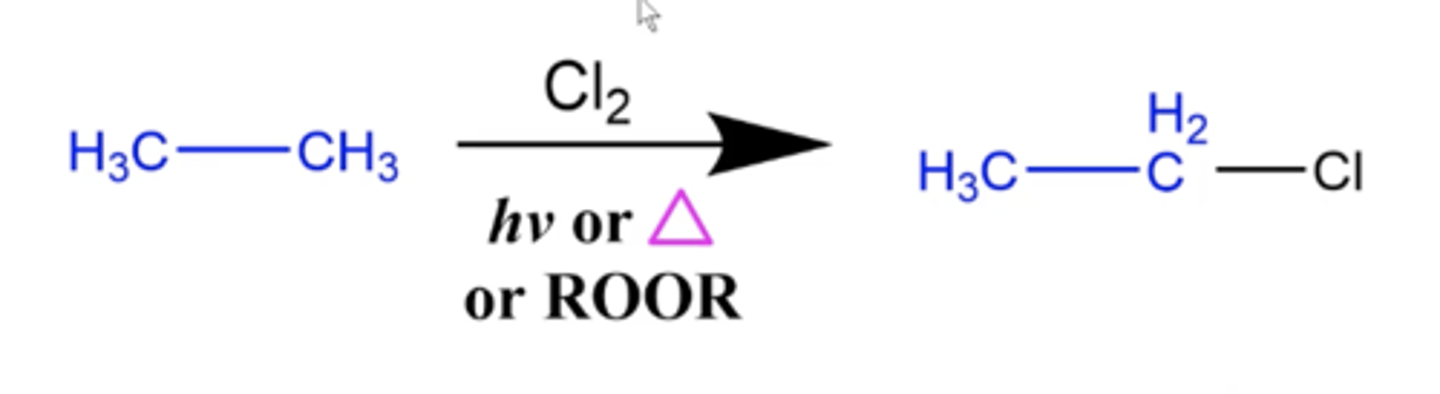
what happens when you add Cl2 plus hv or ROOR to an alkane?
Alkane halogenation reaction:
-Cl replaces one of the hydrogens on the alkane
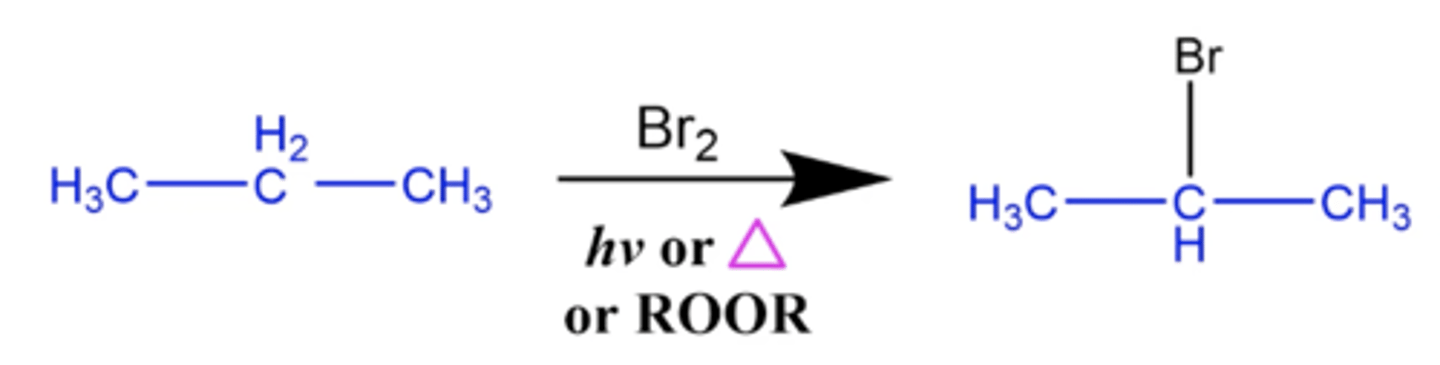
what happens when you add Br2 plus hv or ROOR to an alkane (specifically propane)?
Alkane halogenation reaction:
-one of the central hydrogens on the propane is replaced by Cl
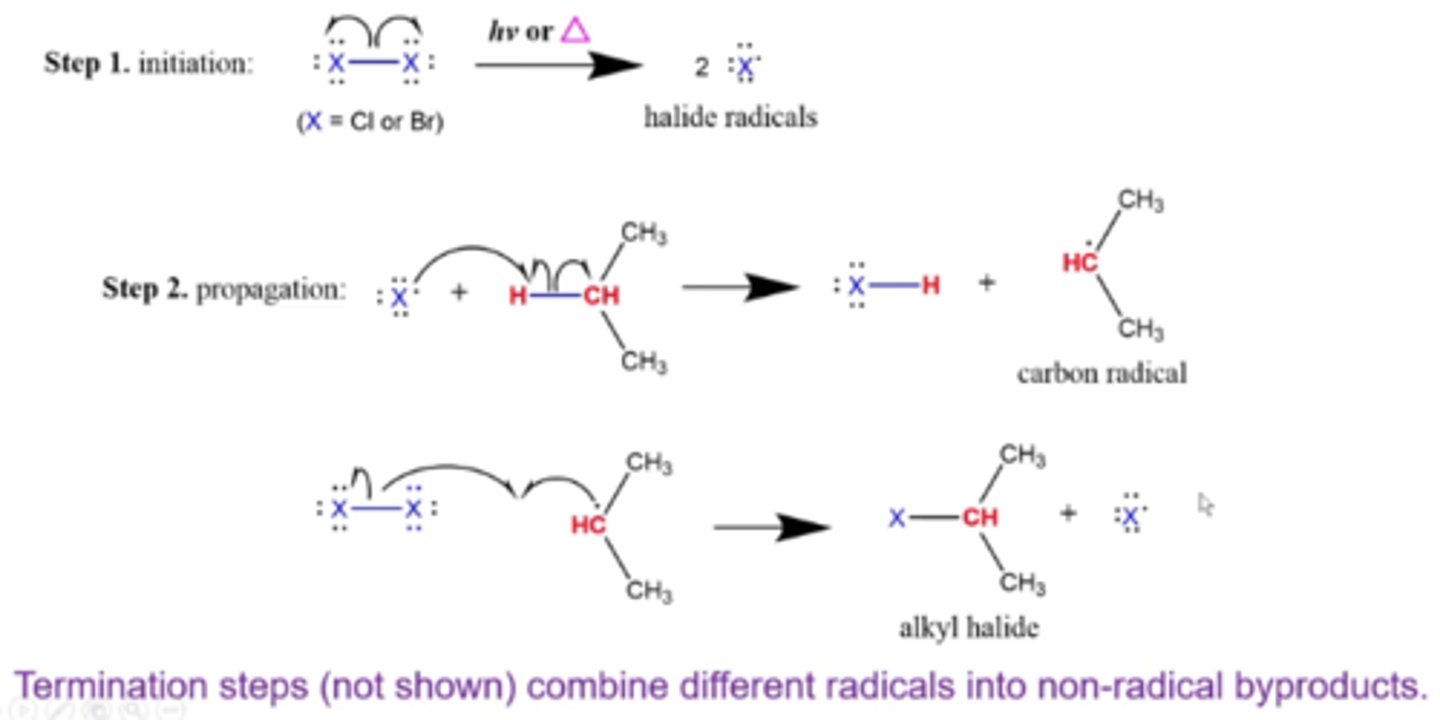
what are the steps of an alkane halogenation reaction?
step 1: initiation (step that does not have radicals on the left side of the equation, but does have radicals on the right side)
step 2: propagation (any step that has one or more radicals on both sides of the equation)
step 3: termination (step that has one or more radicals on the left side, but does not have radicals on the right side)
what is the general mechanism of radical halogenation reactions?
1. halogen breaks up when light hits it, forming 2 halide radicals
2. 1 halogen radical pairs with a hydrogen in the alkane, forming a carbon radical at the most substituted internal carbon
3. a second halogen radical forms a bond with the internal carbon with the previous radical
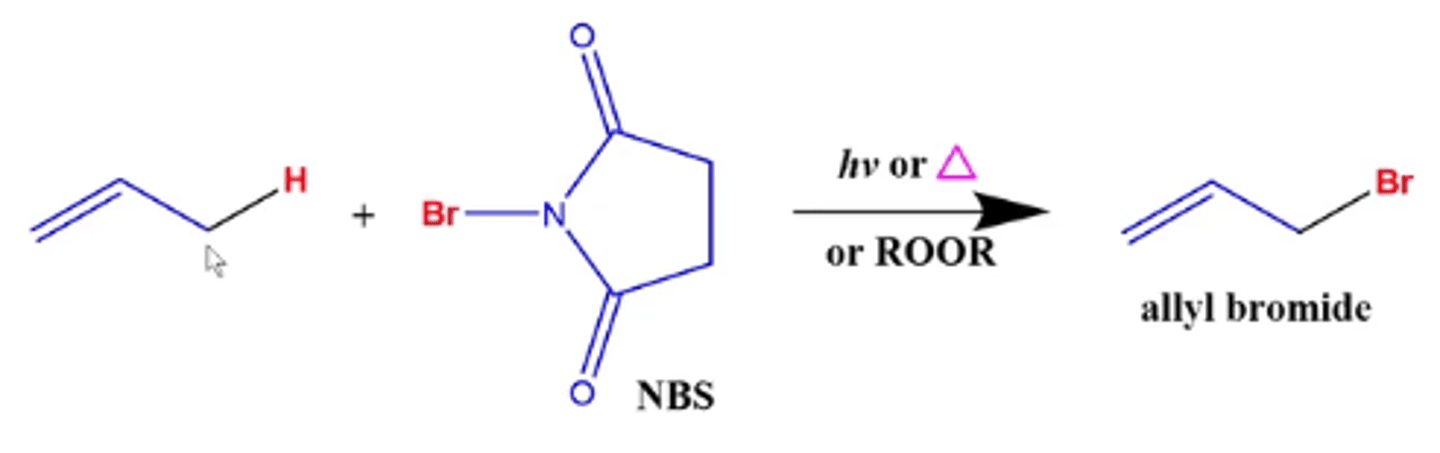
what happens if you take a alkene and react it with NBS plus light or peroxide (ROOR)?
the hydrogen gets replaced by Br
-NBS adds a bromine to a carbon that's one position away from the double bond (the allylic carbon) because it's where the most stable radical is located
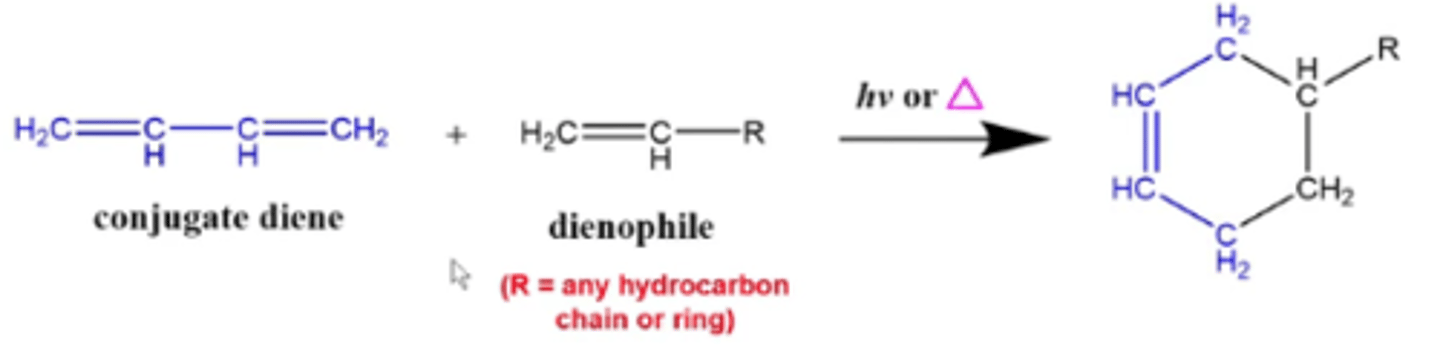
what happens when you react a conjugate diene with an alkene dienophile in the presence of heat or light?
you perform a diels-alder cycloaddition reaction:
-the reactants form a ring with each other, with one double bond inside
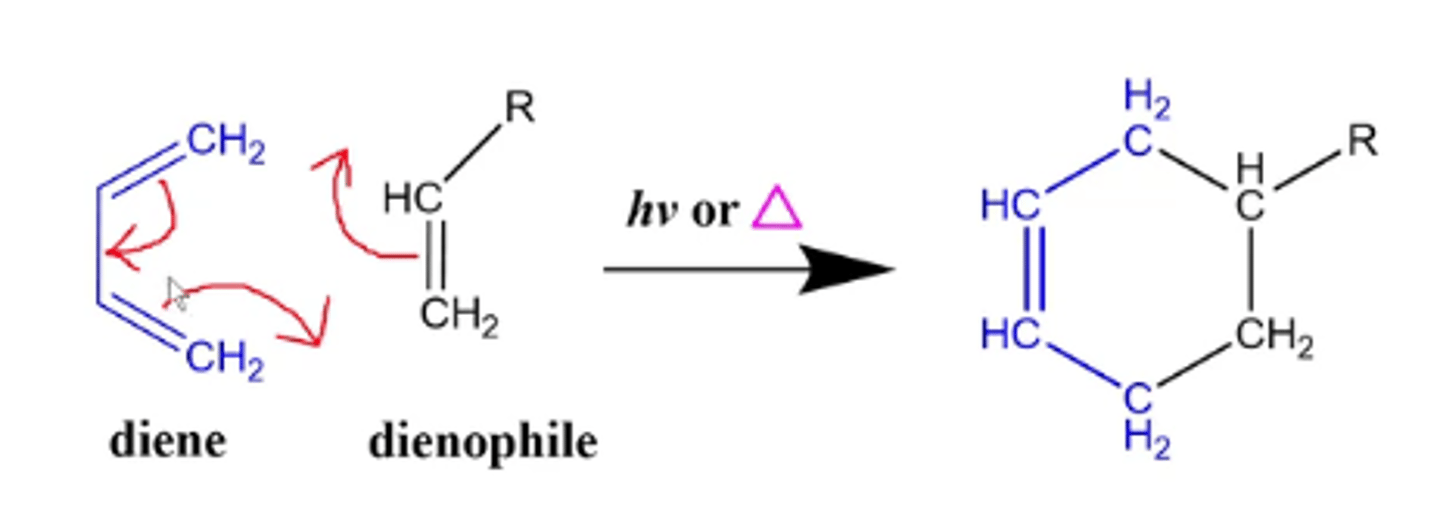
what is the general mechanism of diels-alder cycloaddition reactions?
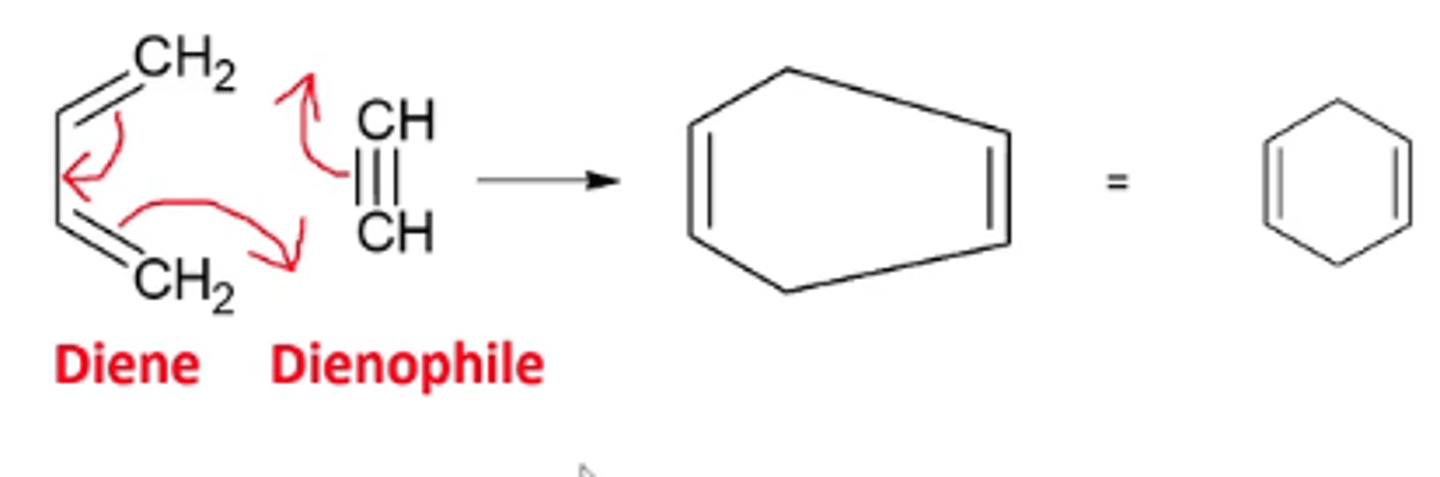
what happens when you react a conjugate diene with an alkyne dienophile?
you get a ring with two opposing double bonds
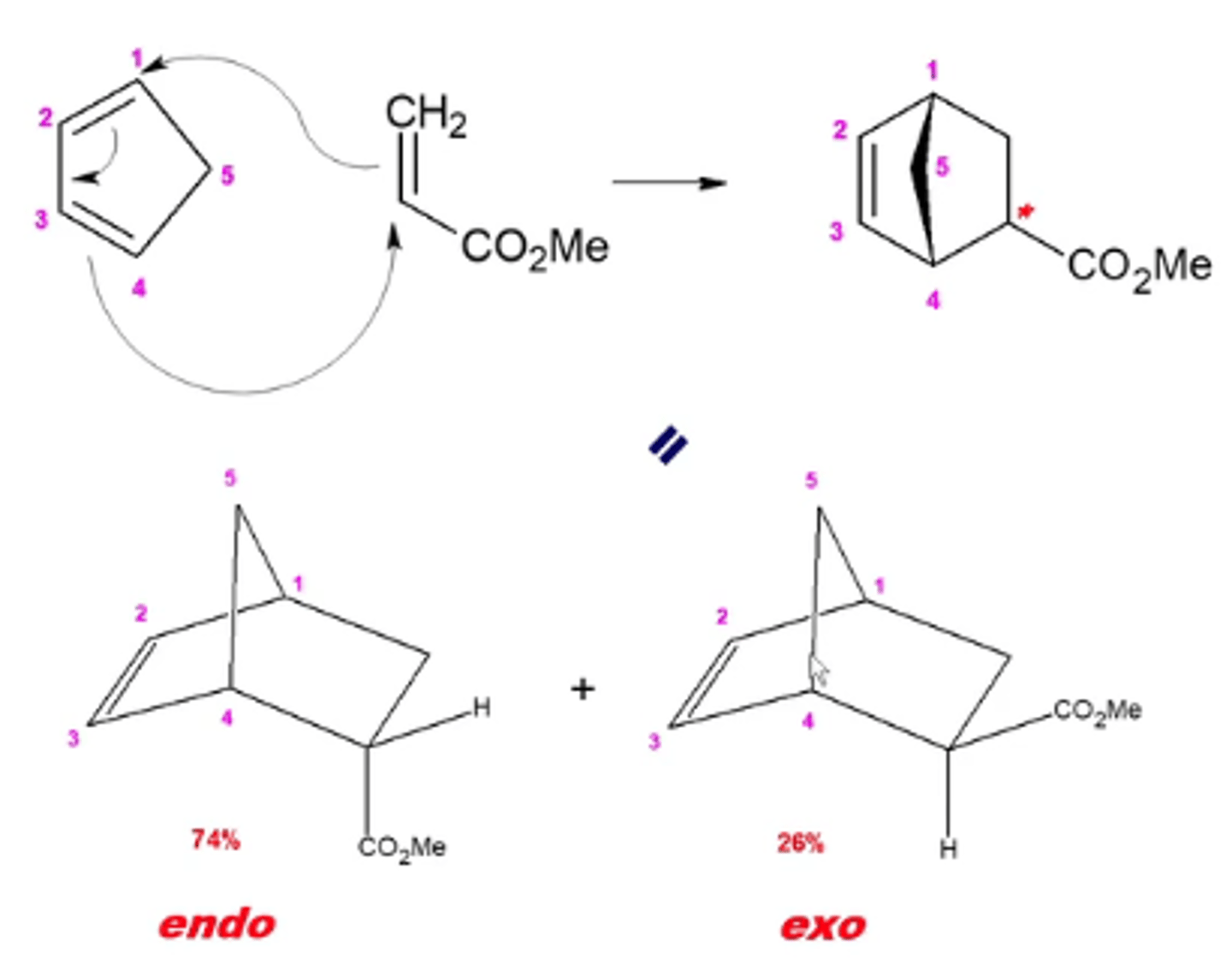
what happens when you react a cyclic conjugate diene with a dienophile?
you perform a diels-alder reaction with cyclic dienes:
this forms a bicyclic ring
-there will be a mix of two products; with the R group facing away from the bridge carbon being the major product (endo) and the minor product being the R group that points toward the bridge carbon (exo)