To study the effect of temperature on reaction rate using sodium thiosulfate and hydrochloric acid
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
7 Terms
Theory
Sodium thiosulfate and hydrochloric acid react to produce a precipitate of sulfur as follows:
Na2S2O3 (Limiting) + 2HCl (excess) → 2NaCl + SO2 + S + H2O
• A solution of sodium thiosulfate are prepared and reacted against a dilute hydrochloric acid solution at different temperatures
• For each concentration, the time taken for a certain mass of the sulfur precipitate to form is recorded and the rate of reaction measured
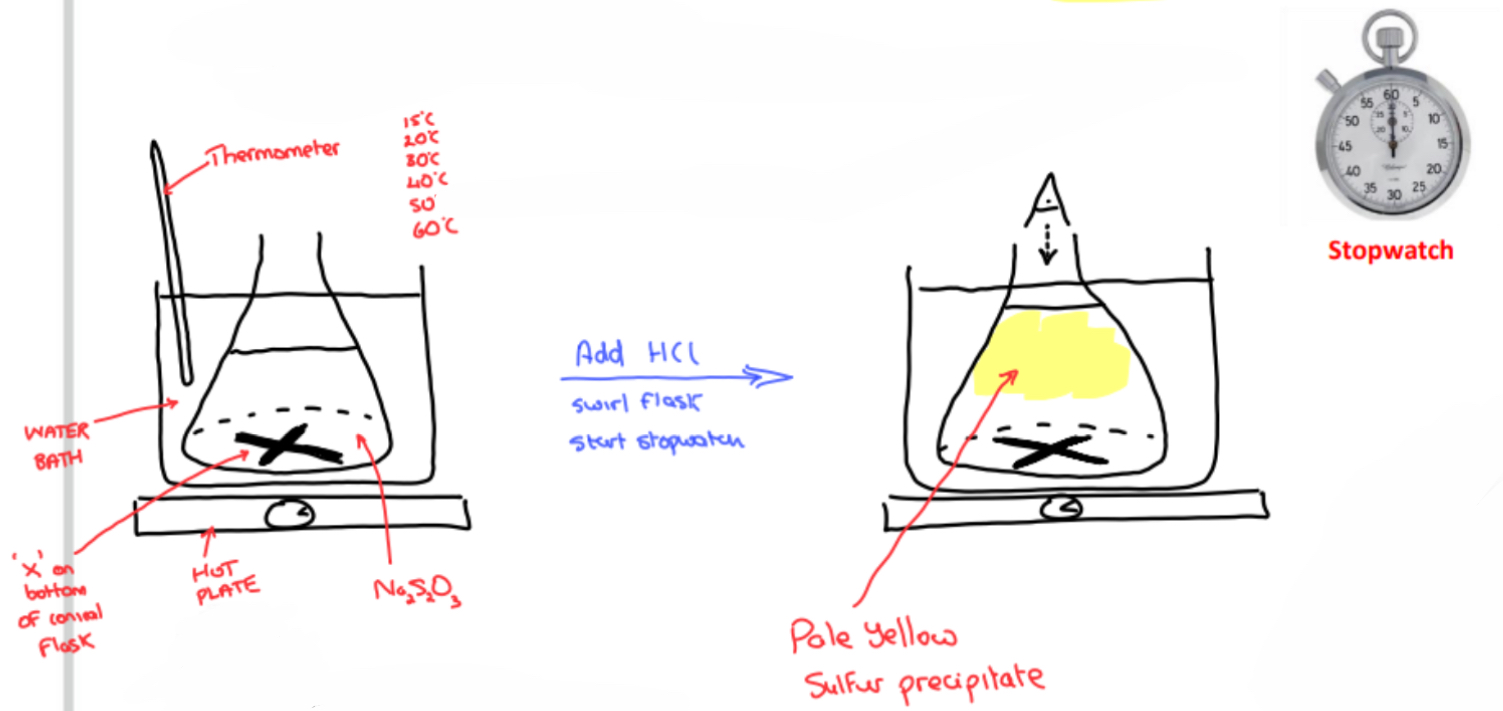
Procedure
➢ Using a graduated cylinder, add a fixed volume of 0.5 M sodium thiosulfate is added to a conical flask
➢ The conical flask has a cross (‘X’) drawn on the bottom with a marker
➢ The conical flask is placed in a water bath on a hot plate and using the thermometer set to a specific temperature
➢ Using another graduated cylinder, a fixed volume of dilute hydrochloric acid is quickly added to the sodium thiosulfate solution in the conical flask
➢ The conical flask is swirled, and the stopwatch is immediately started
➢ While looking down through the solution, the time for it takes for the yellow sulfur precipitate to obscure the cross is recorded
➢ Using a hot plate, water bath and thermometer, the experiment is repeated five times at DIFFERENT TEMPERATURES
➢ The results are entered in a table and a graph of rate (1/time) Vs temperature is plotted
Conclusion
As temperature increases, rate of reaction increases exponentially
Notice: As the graph is not a straight-line graph it is not a directly proportional relationship- rate increases exponentially with temperature
Explain the relationship between rate of reaction and temperature.
• As temperature increases, rate of reaction increases exponentially
1.
- Increasing the temperature increases the energy of the particles
- Higher energy means more effective collisions - more collisions reach the required activation energy
2.
- Increasing the temperature increases the velocity of the particles
- Higher velocity means greater frequency of collisions will occur
- Greater frequency of collisions means more effective collisions - more collisions reach the required activation energy
Note: Exponential means: For every X˚C rise in temperature, rate of reaction increases by a constant factor
(Typically for many reactions – For every 10˚C rise in temperature, rate of reaction doubles)
Why is a water bath preferable to a bunsen burner/directly heating with a hot plate?
It is easier to obtain a desired temperature using a water bath - allows for more gentle heating
Why is it essential the same volume and concentration of hydrochloric acid are used during each reaction?
• Only one variable can be changed in order for the experiment to be a fair test i.e. the concentration of sodium thiosulfate
• All other possible variables must remain fixed
Identify the precipitate in this reaction? Describe its appearance
Sulfur – precipitates as a fine pale-yellow powder