Chemistry - Redox Equilibria
1/37
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
38 Terms
redox equilibria
the instance in which an equilibrium is established between the rate at which a metal loses and gains electrons at its surface is exactly equal
electrochemical cell
a device that converts chemical energy into electrical energy
What are the four main electrochemical cells? (4)
Leclanché dry cell, lead acid accumulator, fuel cell and galvanic cell
Leclanché dry cell
an inexpensive dry cell invented in 1867 by Georges Leclanché in which the cell contains a carbon cathode surrounded by manganese oxide and carbon powder, fueled by an ammonium chloride paste serving as the electrolyte and ion-permeable separator, all of which is encased in zinc which serves as the anode
Describe the functions, longevity and use of the components of the Leclanché dry cell (2/2/1)
The manganese oxide prevents the build-up of hydrogen gas bubbles on the terminal and instead oxidizes it to water at the electrode to maintain efficiency.
The carbon powder increases the surface area of the cathode to increase conductivity.
When the dry cell is being used, the zinc casing becomes thinner as the zinc is oxidized to zinc ions and the electrolyte oozes out of the battery.
It also thins when not in use as the ammonium chloride is acidic and reacts with the zinc to form zinc chloride.
They are often used to power portable electronic devices.
What is the cell diagram of the Leclanché dry cell?
Zn (s) | Zn²⁺(aq) || 2NH₄⁺(aq) | [2NH₃ (g) + H₂ (g)] | C (graphite)
What are the reactions of a Leclanché dry cell? (3)
At the anode: Zn (s) → Zn²⁺ (aq) + 2e⁻
At the cathode: 2MnO₂ (s) + H⁺ (aq) + 2e⁻ → Mn₂O₃ (s) + H₂O (l)
Overall reaction: Zn (s) + 2MnO₂ (s) + 2NH₄⁺ (aq) → Zn²⁺ (aq) + Mn₂O₃ (s) + H₂O (l)
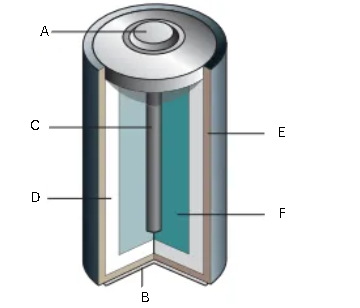
What is the image below? Label it (1/6)
A - Metal cap
B - Metal bottom
C - Carbon rod
D - Moist paste of ammonium chloride
E - Zinc case
F - manganese (IV) oxide
lead acid accumulator
a storage device that serves as a battery in which three or six secondary cells containing a lead anode, a lead (IV) cathode and a sulfuric acid electrolyte generate power
Describe the functions and longevity of the components of the lead accumulators and state its use
When the cell is operated, the lead anode dissolves to form lead (II) ions. At the positive terminal, the lead (IV) oxide cathode reacts with the hydrogen ions in the sulfuric acid to form lead (II) ions and water. The lead (II0 ions formed react with the sulphate ions in the acid to form lead (II) sulphate.
As the lead (II) sulphate precipitates, it coats both the electrodes which slowly reduces the efficiency of the battery. The effect of the precipitation can be undone by recharging the battery in which the lead (II) sulphate is converted back into lead on the anode and lead (IV) oxide on the cathode and is done through the alternator of the car which passes an electric current through the battery in the opposite direction of the cell reactions. However, as the battery discharges for a long time, the concentration of sulfuric acid decreases and the precipitate becomes irreversible and recharging will not restore the battery
They are used to generate power to start car motors
What is the cell diagram of the lead acid accumulator?
Pb(s)|PbSO₄(s)|H₂SO₄ (aq)||H₂SO₄(aq)|PbSO₄(s)|PbO₂(s)
What are the reactions of a lead acid accumulator? (3)
At the anode: Pb (s) + SO₄²⁻ (aq) + PbSO₄ (s) + 2e⁻
At the cathode: PbO₂ (s) + 4H⁺ (aq) + SO₄²⁻ (aq) + 2e⁻ → PbSO₄ (s) + H₂O (l)
Overall reaction: Pb (s) + PbO₂ (s) + 2H₂SO₄ (aq) → 2PbSO₄ (s) + H₂O (l)
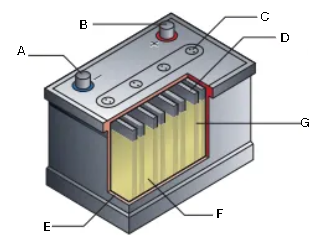
What is this diagram? Label it (1/7)
A - negative terminal
B - positive terminal
C - gas vents
D - lead
E - insulating case
F - sulfuric acid
G - lead oxide
fuel cell
those in which hydrogen, hydrocarbons or alcohols are used a source of chemical energy and electricity is generated when an oxidizing agent reacts with the fuel supply
Describe the functions and longevity of a hydrogen/oxygen fuel cell
Hydrogen is the fuel and oxygen is the oxidizing agent while the anode and cathode are porous graphite electrodes coated in nickel or platinum catalysts and the electrolyte is warm potassium hydroxide solution. In the cell, the hydrogen gas enters the negative compartment and diffuses through the porous anode after which the catalyst breaks it down into aqueous hydrogen ions and electrons. The hydrogen ions enter the KOH solution where they combine with the hydroxide solutions to form water as a waste chemical. The electrons flow through the external circuit to the cathode and create an electric current. The oxygen gas enters the positive compartment and diffuses through the porous cathode where it combines with water and electrons to form hydroxide ions.
The fuels in fuel cells must be replenished in order for it to function continuously but they have the advantage of having a 45% efficiency
What is the cell diagram of the hydrogen/oxygen fuel cell
C (graphite, Ni)|H₂(g)|OH⁻(aq)|OH⁻(aq)|O₂(g)|C(graphite, Ni)
What are the reactions in a hydrogen/oxygen fuel cell? (3)
At the anode: H₂ (g) + 2OH⁻ (aq) → 2H₂O (l) + 4e⁻
At the cathode: O₂ (g) + 2H₂O (l) + 4e⁻ → 4OH⁻
Overall reaction: O₂ (g) + 2H₂ (g) → 2H₂O (l)
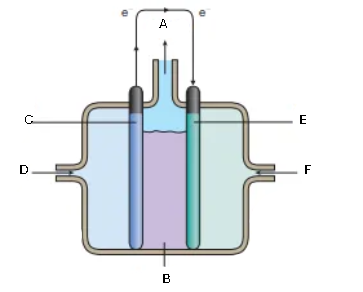
What is this diagram? Label it (6)
A - water
B - KOH
C - Carbon anode (Ni)
D - hydrogen gas
E - Carbon cathode (Ni and NiO)
F - oxygen gas
galvanic cell
an electrochemical cell that is designed to convert the chemical energy of spontaneous redox reactions into electrical energy, consisting of two half cells each containing an electrode, an aqueous electrolyte and a salt bridge and external circuit connecting the two
Describe the functions of a metal-metal ion galvanic cell
The more reactive metal will become the anode and will be oxidized resulting in a loss of electrons and ionization. The electrons will flow through the circuit to the other electrode (cathode) where the ions in solution will be reduced. The anions in the salt bridge flow to the anode half cell in order to offset the accumulation of positive charge and the cations in the salt bridge flow to the cathode half cell to offset the accumulation of negative charge. This is done so that the flow of electrons can continue.
Which salts can be used in a salt bridge? (3)
Potassium nitrate, potassium chloride and sodium chloride
What is the cell diagram of a zinc/copper galvanic cell?
Zn (s)|Zn²⁺(aq)||Cu²⁺(aq)|Cu(s)
What are the reactions of a zinc/copper galvanic cell? (3)
At the anode: Zn (s) + 2e⁻ → Zn²⁺ (aq)
At the cathode: Cu²⁺ (aq) + 2e⁻ → Cu (s)
Overall reaction: Zn (s) + Cu²⁺ (aq) → Zn²⁺ (aq) + Cu (s)
electrode potential
the potential difference between an electrode and a solution of its ions relative to another electrode
cell potential (2)
the potential difference between two half-cells
electromotive force
standard electrode potential
the potential of a half cell relative to the standard hydrogen electrode under standard conditions
Why was the standard electrode potential developed?
The electrode potential of a metal and its dissolved ions is related to the amount of voltage its half-cell produces when the metal loses or gains electrons. This is unreliably comparable when comparing the potential difference between an electrode and its dissolved ions against an arbitrary electrode and hence a standard was developed in order to compare the electrode potential and this is called the standard electrode potential.
Describe standard conditions (3)
Gases at 1 atm pressure
1M solutions
298K temperature
standard hydrogen electrode (SHE)
a galvanic cell configuration that consists of hydrogen gas at 1 atm pressure in equilibrium with an aqueous solution of hydrogen ions at a concentration of 1M and a platinum electrode coated in platinum black that contacts both the hydrogen gas and its ion
Describe and state the reaction in the SHE
Hydrogen gas bubble around the platinum and adsorb on its surface and the equilibrium is established between the adsorbed layer of gas and aqueous ions. It has an electrode potential of 0 at all temperatures.
Reaction: 2H⁺ (aq) + 2e⁻ ⇌ H₂ (g)
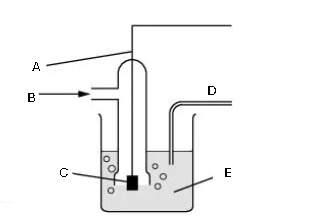
What is this diagram? Label it (5)
A - platinum wire
B - hydrogen gas
C - Pt black
D - salt bridge
E - hydrogen ions in solution
How do we determine the standard electrode potential?
Under standard conditions, the EMF is the standard electrode potential and can be found using the formula E°= E(positive electrode) - E(negative electrode)
How can we use the standard electrode potential to determine the flow of electrons?
The half-cell with the lower standard electrode potential will supply the electrons
Describe the procedures for measuring the standard electrode potential of a half-cell containing non-metals and their ions and ions of the same element in different oxidation states (1/1)
When measuring the standard electrode potential of a half-cell containing non-metals and their ions, an inert electrode must be in contact with the element and a solution of its ions.
When measuring the standard electrode potential of a half-cell containing ions of the same element in differing oxidation states, the half-cell containing the ions must have solution containing both ions, each at a concentration of 1M.
standard cell potential
difference in potential of two standardized half-cells under standard conditions
What is the standard cell potential used for?
Measuring the voltage an electrochemical cell
How can we use standard cell potential to determine the feasibility of a reaction?
A negative standard cell potential indicates that a redox reaction between the elements in the half-cells is not feasible energetically. We calculate this by substituting the standard electrode potentials of each half cell into the formula for standard cell potential
How does the value of a standard electrode potential change when the concentration of ions decrease and why?
When the concentration of a metal decreases in a metal-metal ion system, the standard electrode potential becomes more negative as the decrease in the ions results in the release of more electrons in order to restore equilibrium.