Chemistry - Unit 10 -Solutes/Solubility
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
Solute
The substance that is dissolved in a solution.
Solvent
The substance that is doing the dissolving, typically water.
Solution
A homogenous mixture of solute and solvent.
Saturated Solution
A solution that contains the maximum amount of dissolved solute for a given amount of solvent.
-You cn see some undissolved solute at the bottom (heterogenous)
Unsaturated Solution
A solution that contains less than the maximum amount of dissolved solute for a given amount of solvent.
-you can still addmore solute to the solution.
-you cannot see the solute, it is FULLY DISSOLVED (homogenous)
Temperature and Solubility - Solids in Liquid
As temperature increases, the solubility increases
Solvent molecules interact with solute more frequently to separate the ions (overcome IMFS)
As temperature decreases, the solubility decreases
Solvent molecules interact with solute less frequently to separate the ions (overcome IMFs)
DIRECT RELATIONSHIP, ONE INCREASES THE OTHER INCREASES AND VICE VERSA
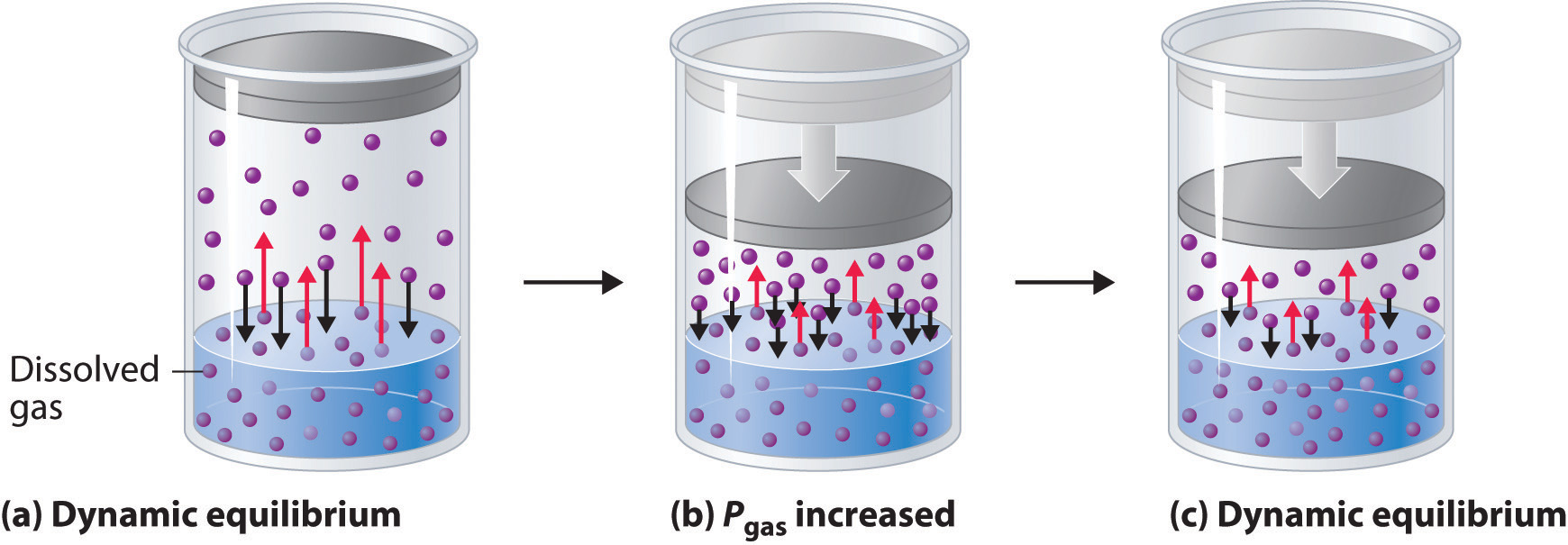
Temperature and Solubility - Gases in Liquid
As the temperature increases, the solubility of a gas decreases
Gas particles move more, take up more space and escape the liquid
As the temperature decreases the solubility of a gas increases
Gas particles move less, taking up less space, and stay dissolved in the liquid
INDIRECT RELATIONSHIP, ONE INCREASES THE OTHER DECREASES AND VICE AND VERSA*
How to Use Table G
Table G is titled as Solubility Curves at Standard Pressure. The x-axis title is Temperature and the y-axis is Solubility (g solute/100. g H2O). This chart shows us how much of each substance dissolves in 100g of water, emphasis on 100 grams, at different temperatures.
MAKE SURE TO CHANGE THE AMOUNT OF WATER TO 100G (EX: if its out of 200 multiply maximum solute by two)
NOTICE:
Gases lines decrease as temperature increases
Solid lines increase as temperature increases
Crystallization
The process where a crystal precipitate forms from a liquid.
in a saturated solution the rate at which a solid dissolves is equal to or greater than the rate at which is crystallizes
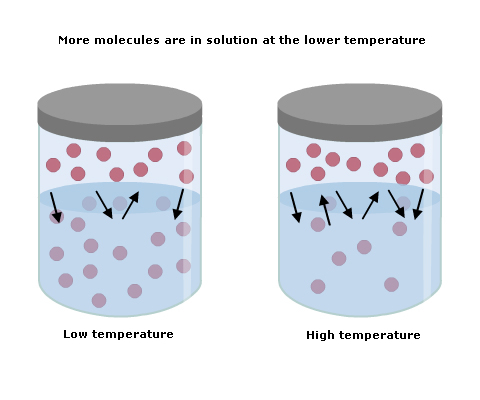
Supersaturated Solution
More than the maximum amount of solute is dissolved in a solvent at a given temperature
Solution needs to be heated up and then cooled down to make a supersaturated solution
LESSON
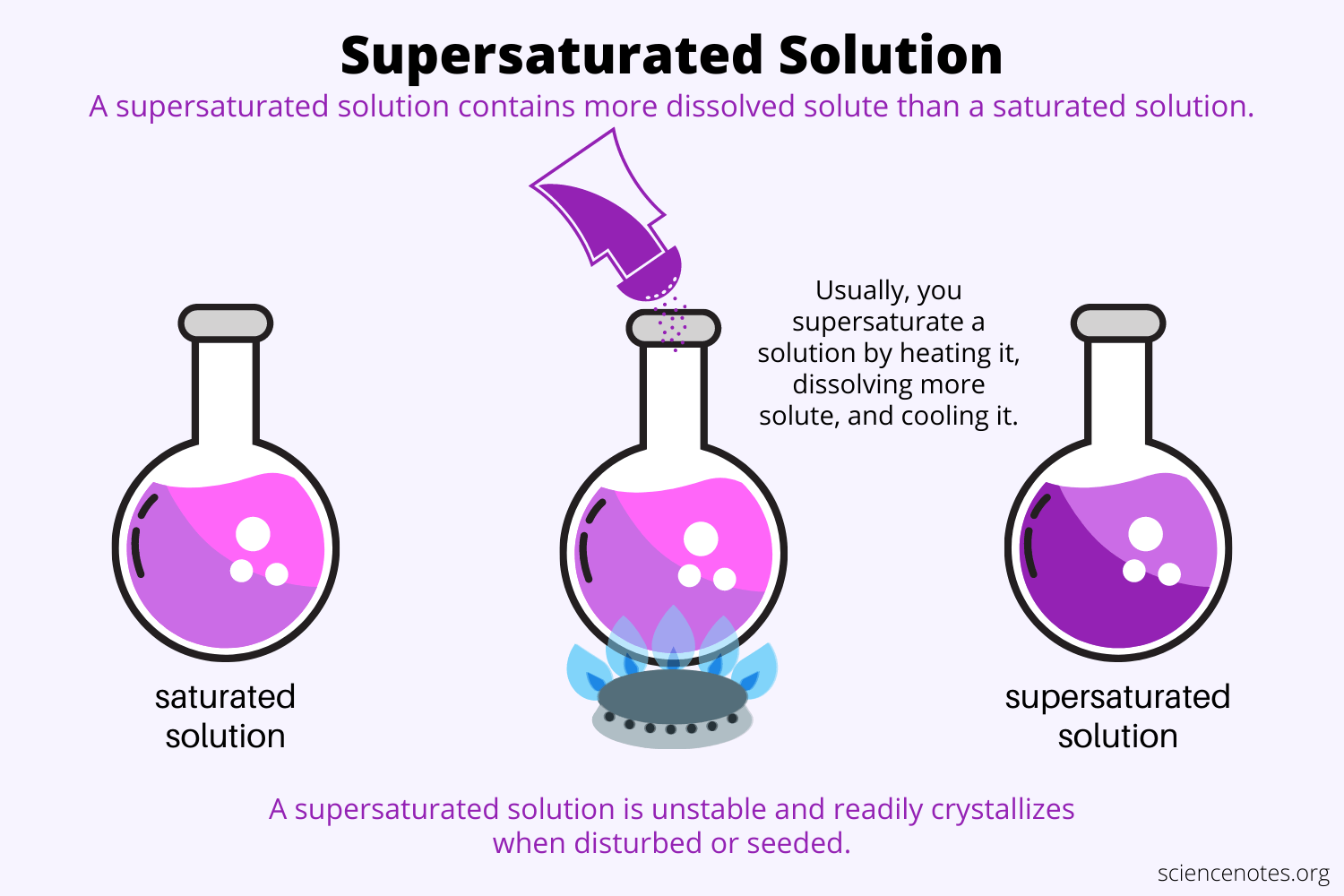
How to Increase gas solubility
Temperature decrease: increases gas solubility, particles move less and don’t escape
Pressure increase: Prevents gas particles from escaping container
How to decrease gas solubility
Temperature increase: dncreases gas solubility, particles move more and escape
Pressure decrease: Less prevention of gas particles from escaping container (more escape)
How to Increase solid solubility
Increase solid solubility
Temperature increase: increases solid solubility, more energy added to break apart bonds
PRESSURE HAS NO EFFECT ON THE SOLUBILITY OF SOLIDS BECAUSE THE SOLID LATTICE STRUCTURE CANNOT BE COMPRESSED
How to Decrease solid solubility
Temperature decrease: decreases solid solubility, less energy added to break apart bonds
PRESSURE HAS NO EFFECT ON THE SOLUBILITY OF SOLIDS BECAUSE THE SOLID LATTICE STRUCTURE CANNOT BE COMPRESSED
Does pressure impact the solubility of solids?
NO! Particles are in a crystal lattice structure and cannot be further compressed, thus it cannot impact the solubility of solids.

All ways to change the rate of solubility
STIRRING/MIXING: The more you physically mix a solution (increase particle interactions) the faster the solute dissolves
Particle Size/Surface Area: the smaller the particles, the greater amount of total surface area. More surface area = more interaction between solute and solvent particles and faster dissolving
Polarity: solute and solvent will only mix if they have similar polarity (polar and polar, non polar and non polar, like mixes with like)
Rate of Solubility
How FAST something dissolves, not effect on the AMOUNT that can be dissolved ( solubility )
Does stirring/mixing impact solubility?
NO! It only impact the RATE of solubility, how FAST something dissolves not how much. (More particle interaction, faster dissolving)
Concentration
the amount of substance (solute) in a given amount of solvent
Tells us the specific amount of solute in a solvent (quantitative)
Concentrated and dilute are not specific (qualitative) and just tells us GENERALLY if solutions have higher/lower concentrations
Molarity (M)
the amount of substance (solute) in a given amount of solvent
Tells us the specific amount of solute in a solvent (quantitative)
Concentrated and dilute are not specific (qualitative) and just tells us GENERALLY if solutions have higher/lower concentrations
Molarity: a measure of concentration noted in the moles per liter followed by a M (ex: #M)
Moles solute/Liters solution M= m/L (moles per liter)
Parts Per Million (PPM)
A measure of concentration calculated as mass of solute per mass of solvent multiplied by 1,000,000.
Solute/solution x1,000,000
Boiling Temperature
The temperature at which a substance transitions to gas; increases when solute is added.
Freezing Point
The temperature at which a substance transitions to solid; decreases when solute is added.
Colligative properties
Colligative properties are the physical changes that result from adding solute to a solvent. Colligative Properties depend on how many solute particles are present as well as the solvent amount, but they do NOT depend on the type of solute particles, although do depend on the type of solvent.
When solute is added to a solvent particles of solute get in the way of the solvent molecules transitioning to the gas phase
VAPOR PRESSURE DECREASES AS SOLVENT IS ADDED
Because transitioning to gas phase is now harder with lower vapor pressure (less molecules being able to escape due to pressure) the kinetic energy required to phase change to gas increases, increases KE required means higher boiling temp
BOILING TEMPERATURE INCREASES AS SOLVENT IS ADDED
Solute interferes with the ability of solutions to form the solid lattice of crystal structure
FREEZING POINT OF SOLUTION DECREASES AS SOLVENT IS ADDED
The more concentrated the solution, the larger the effect on the freezing point AND boiling point, again more energy is needed to be lost or added to phase change due to the annoying solvent particles being in the way
MORE CONCENTRATED = BIGGER IMPACT (INCREASE IN BOILING, DECREASE IN FREEZING)
The more particles in a solution (ions or molecules) the larger the effect on the freezing and boiling point
MORE PARTICLES = BIGGER IMPACT (INCREASING BOILING POINT, DECREASING FREEZING POINT)
As solvent is added: particles get in the way so phase change requires more energy leading to…
VAPOR PRESSURE DECREASE,
BOILING TEMPERATURE INCREASE,
FREEZING POING DECREASE
On a particle level why and how does solute impact freezing and boiling point?
They get in the way of phase change requiring more energy lost or gained for phase change. THUS, increased boiling point, decreased freezing point.
The intensity of the impact can be effected by…
More concentrated (look at molartiy) = more particles per liter of solution, more annoying particles = more energy needed for phase change
More particles = more annoying particles getting in the way, more energy needed to phase change
How to tell if a compound will dissolve into ions in water and what ions it will dissolve into
To determine if a compound will dissolve into ions in water, check if it is ionic or highly polar covalent. Ionic compounds and some polar covalent compounds (like acids) typically dissociate into ions. To know what ions it will dissolve into, identify the cation and anion based on the compound's formula (e.g., NaCl will dissolve into Na+ and Cl- ions).
How to determine the cation and anion a compound will dissociate into
To determine the cation and anion that a compound will dissociate into, check if the compound is ionic or highly polar covalent. Then, identify the cation (positive ion) and anion (negative ion) based on the compound's formula. For example, in sodium chloride (NaCl), Na+ is the cation and Cl- is the anion.
How to tell if a compound will dissociate in water
To determine if a compound will dissociate in water, check if it is an ionic compound or a highly polar covalent compound (like acids). Ionic compounds typically dissociate into their respective ions due to strong electrostatic forces.