BISC 1111 Exam Review Slides
1/100
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
101 Terms
Seven characteristics of life
cellular organization
ordered complexity
sensitivity
growth, development, and reproduction
energy utilization
homeostasis
evolutionary adaptation
Cellular organization
all life is composed of one or more membrane bound cells
Ordered complexity
molecules and cells making up an organism exhibit hierarchical, ordered complexity
Sensitvity
respond to stimuli
Growth, development, and reproduction
governed by molecules of heredity
Energy utilization
metabolism, take in energy that is used for work
Homeostasis
maintain relatively stable internal conditions different from external environment
Evolutionary adaptation
organisms interact with one another and their environment in ways that influence survival, and therefore, populations evolve to adapt to their environments
Theory to lay person
a theory to the general public usually implies uncertainty; a lack of knowledge or a guess
Theory to a scientist
a proposed explanation for some natural phenomenon, often based on some general principle
body of interconnected concepts, supported by scientific reasoning and experimental evidence, that explains the facts in some area of study
Deductive reasoning
applies general principles to predict specific results
used to test validity of ideas in all branches of science, but especially in mathematics and philosophy
can be used to infer the species of a specimen from its characteristics
Inductive reasoning
uses specific observations to construct a general set of principles (logic flow opposite of deductive reasoning)
leads to generalizations that can then be tested
Reductionism - A philosophical approach to understand a complex system by reducing it to its working parts.
Hypothesis
suggested explanations that account for observed phenomena
a set of propositions which might be true
Natural selection
the differential reproduction of genotypes; caused by factors in the environment; leads to evolutionary change
Chemical behavior is determined by
the distribution of electrons in the electron shells
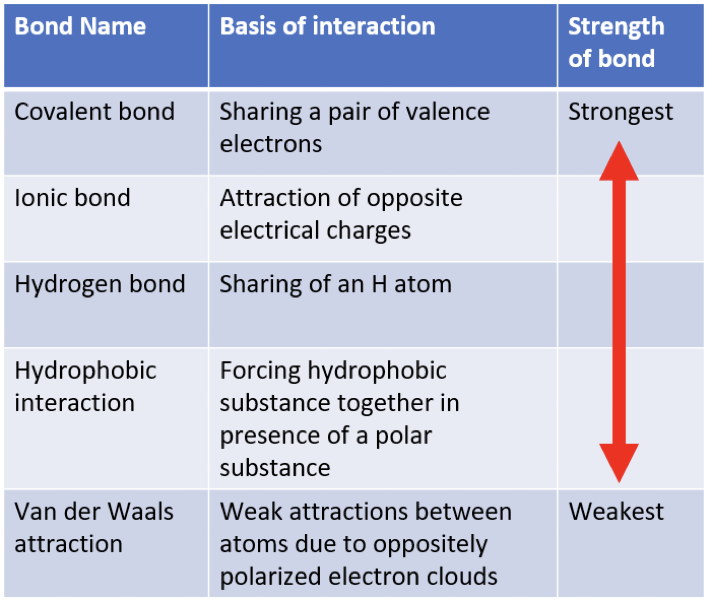
Covalent bonds
the sharing of a pair of valence electrons by two atoms
shared electrons count as part of each atom’s valence shell
Electronegativity
an atom’s attraction for the electrons in a covalent bond
Nonpolar covalent bond
the atoms share the electron equally
always occurs if the atoms bonded together are the same element (H2, O2, N2) etc…
form between two different atoms of similar electronegatives
Polar covalent bonds
one atom is more electronegative, and the atoms do not share the electron equally
unequal sharing of electrons causes a partial negative or positive charge for each atom or molecule
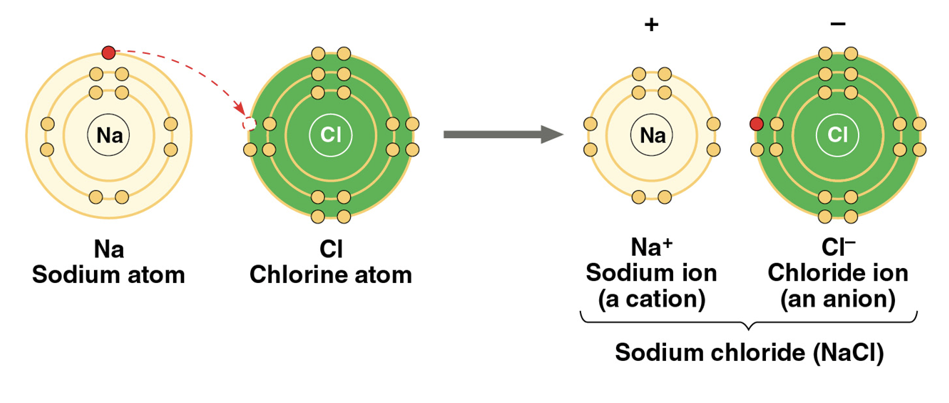
Ionic bond
atoms strip an electron from their bonding partners—attraction of opposite electrical charges
weaker than covalent bond, stronger than hydrogen bond
4 main mechanisms of evolutionary change
mutation - introduction of new alleles
gene flow - movement of genetic information from one population to another
genetic drift - chance event changes allele frequencies
natural selection - the differential reproduction of genotypes; caused by factors in the environment; leads to evolutionary change
Chemical bonds
Reactions are influenced by 3 main factors: temperature
heating reactants increase reaction rates because reactants collide with one another more often
Reactions are influenced by 3 main factors: concentration of reactants & products
proceed faster when more reactants available for collisions. accumulation of product slows or reverses reactions
Reactions are influenced by 3 main factors: catalysts
a substance that increases reaction rates
in living systems, proteins called enzymes catalyze most reactions needed for life… they do largely by bringing reactants into very close proximity
Water’s four emergent properties vital for life
cohesive behavior
ability to moderate temperature
expansion upon freezing
versatility as a solvent
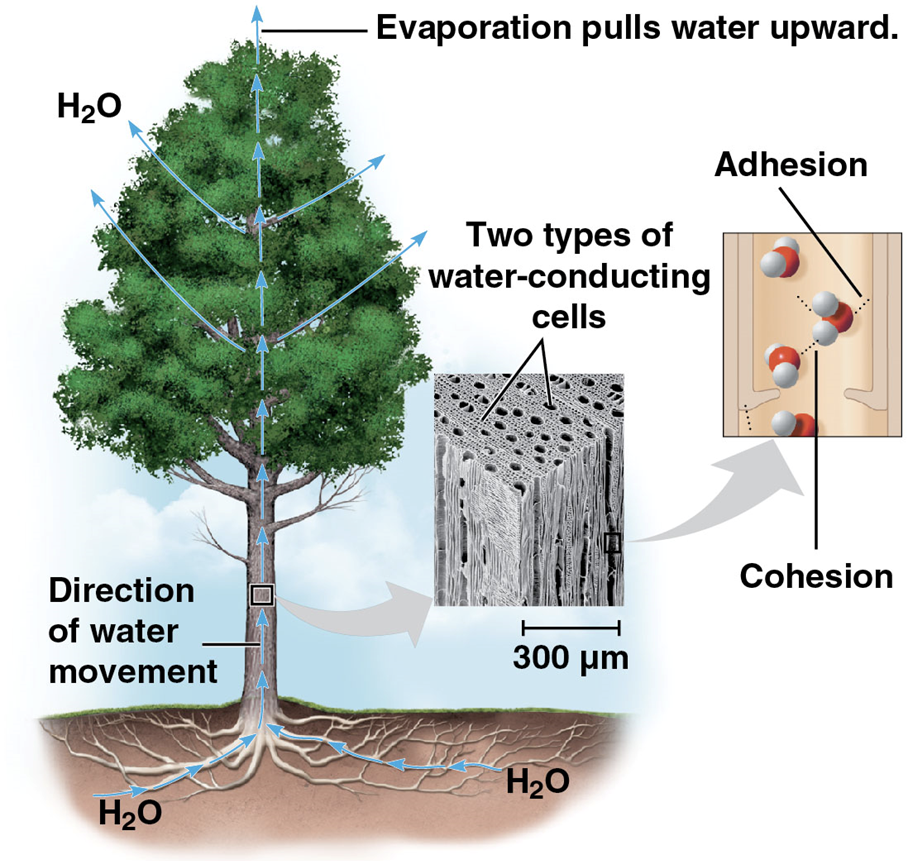
Cohesive behavior
hydrogen bonds collectively hold polar water molecules together
results in surface tension and adhesive properties
cohesion and adhesion (attraction between water and non-water substances)
Hydrogen bonds
forms when a hydrogen atom covalently bonded to one electronegative atom is also attracted to another electronegative atom
in living cells, the electronegative partners are usually oxygen or nitrogen atoms
In the water molecule, the electrons of the _____________ spend more time near the oxygen than the hydrogen
polar covalent bonds
surface tension
a measure of how difficult it is to stretch or break the surface of a liquid
cohesion results in a high __________
Adhesion
an attraction between different substances, for example between water and plant cell walls
helps counter down pull of gravity → upward direction of water movement (transport) in plants
Water moderates temperature
water absorbs heat from warmer air and releases stored heat to cooler air
can absorb or release a large amount of heat with only a slight change in its own temperature
because of water’s high specific heat
Specific heat
the amount of heat that must be absorbed or lost for 1 g of that substance to change its temperature by 1ºC
of water is 1 cal/(g * ºC)
Expansion upon freezing/solid less dense than liquid
ice floats on water because it is less dense than liquid water
water molecules locked into crystalline lattice when freezing where the hydrogen bonds of adjacent molecules keep them far enough apart to make ice ~10% less dense than water
Universal solvent/solvent of life
water is the best solvent because of its highly polar nature
when an ionic compound is dissolved in water, each ion (whichever the electrical charge) is surrounded by a sphere of water molecules called a hydration shell
Hydrophilic substance
substance that has an affinity for water
proteins, carbs, ionic compounds, etc… basically anything polar
Hydrophobic substance
substance that does not have an affinity for water
alkanes, oils, fats, greases, etc… basically anything relatively non-polar
Water freely dissociates into…
hydroxide and hydronium
hydrogen atom leaves its electron behind and is transferred as proton/hydrogen ion (H+)
hydroxide ion (OH-) - molecule at that lost the proton
hydronium ion (H3O+) - molecule with the extra proton, often represented as H+
Acid
a substance that increases the H+ concentration of a solution
HCl → H+ + Cl-
pH value less than 7
Base
a substance that reduces the H+ concentration of a solution
NH3 + H+ → NH4+ (reversible)
NaOH → Na+ + OH-
pH value greater than 7
Buffers
substances that minimize changes in concentrations of H+ and OH- in a solution
most solutions contain a weak acid and a corresponding base, which combine reversibly with H+ ions
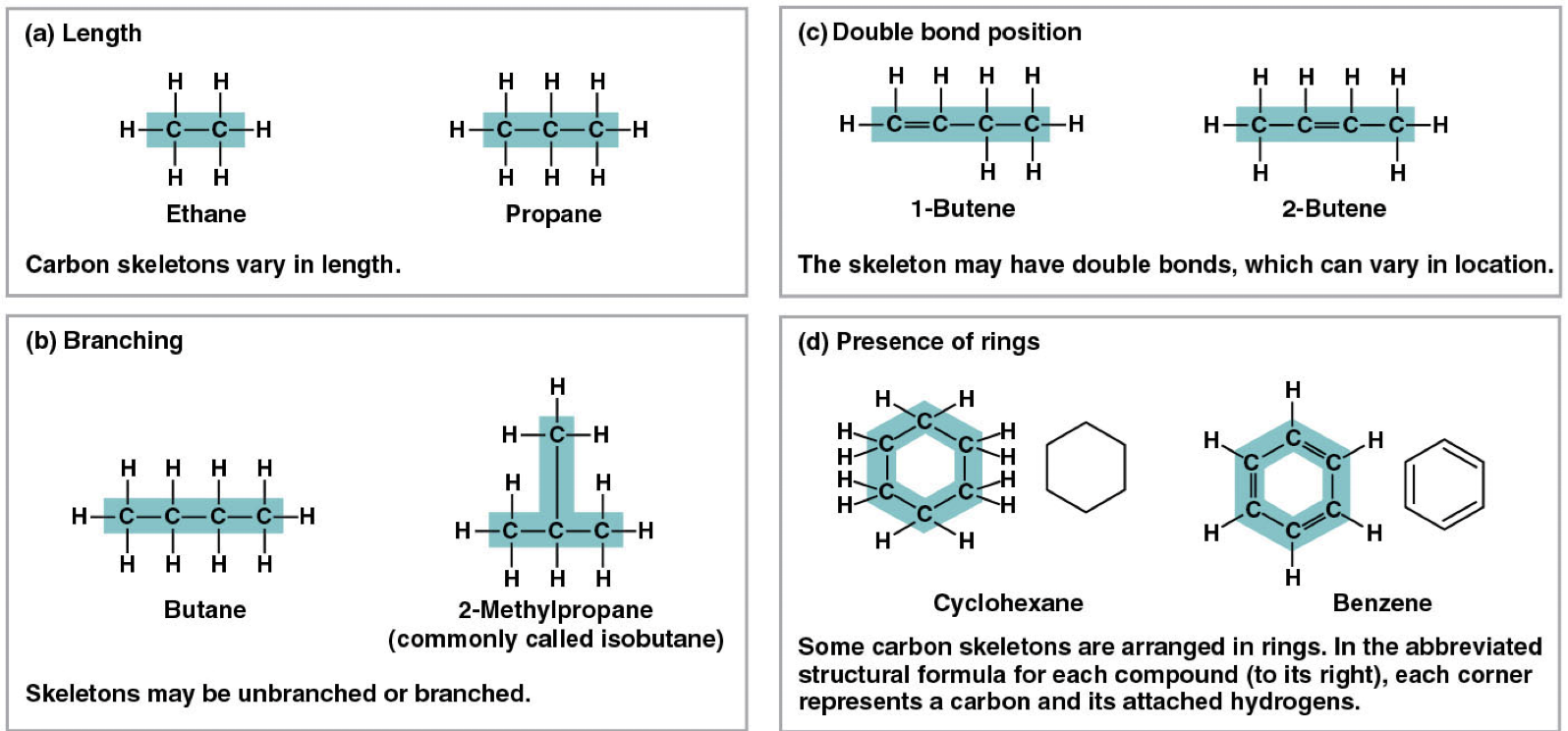
Carbon skeleton variation generates molecular diversity
Carbon skeletons vary in length
The skeleton may have double bonds, which can vary in location
Skeletons may be branched or unbranched
Some skeletons are arranged in rings
Isomers
compounds with the same molecular formula but different structures and properties
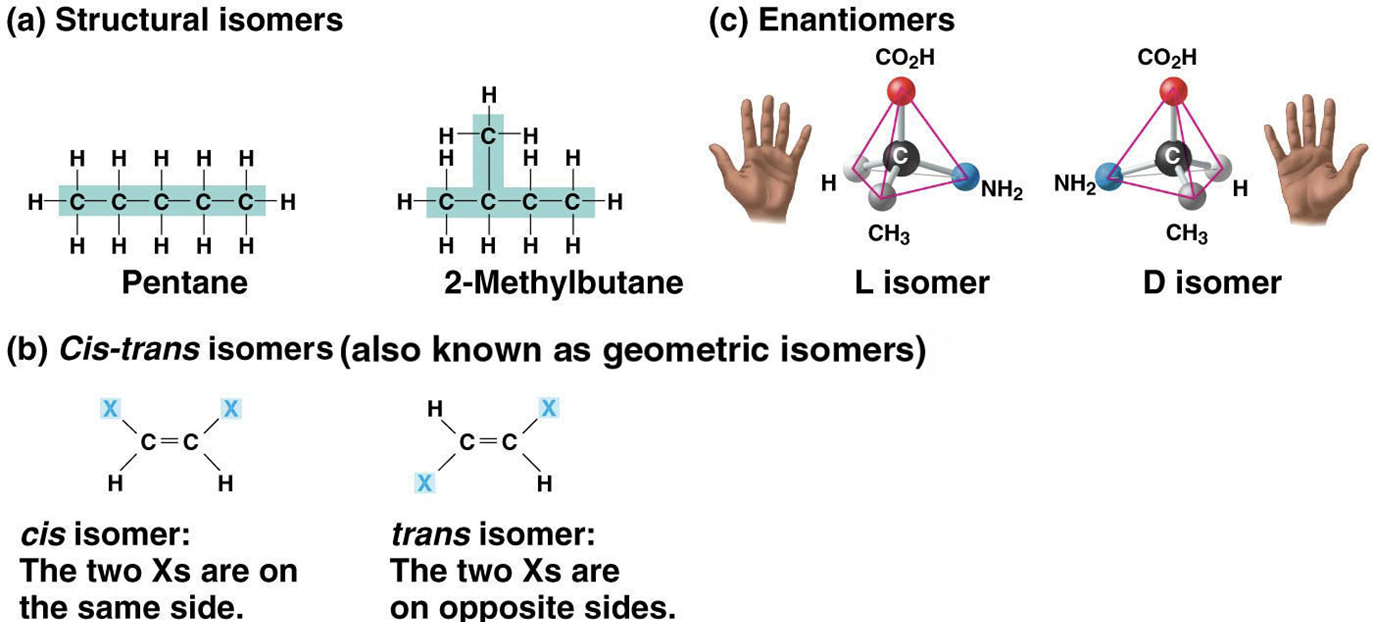
Structural isomers
have different covalent arrangements of their atoms
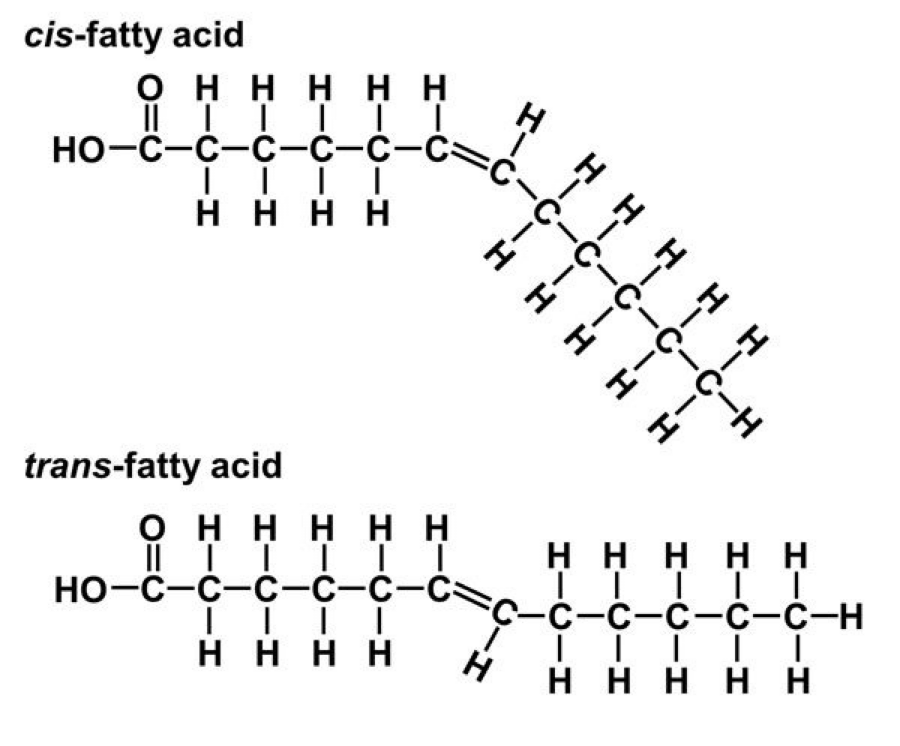
Cis-trans isomers
(also called geometric isomers) have the same covalent bonds but differ in their spatial arrangements
Enantiomers
mirror images of each other
Different enantiomers can cause certain antibodies to be ineffective
ie S-ibuprofen is effective while R-ibuprofen is ineffective
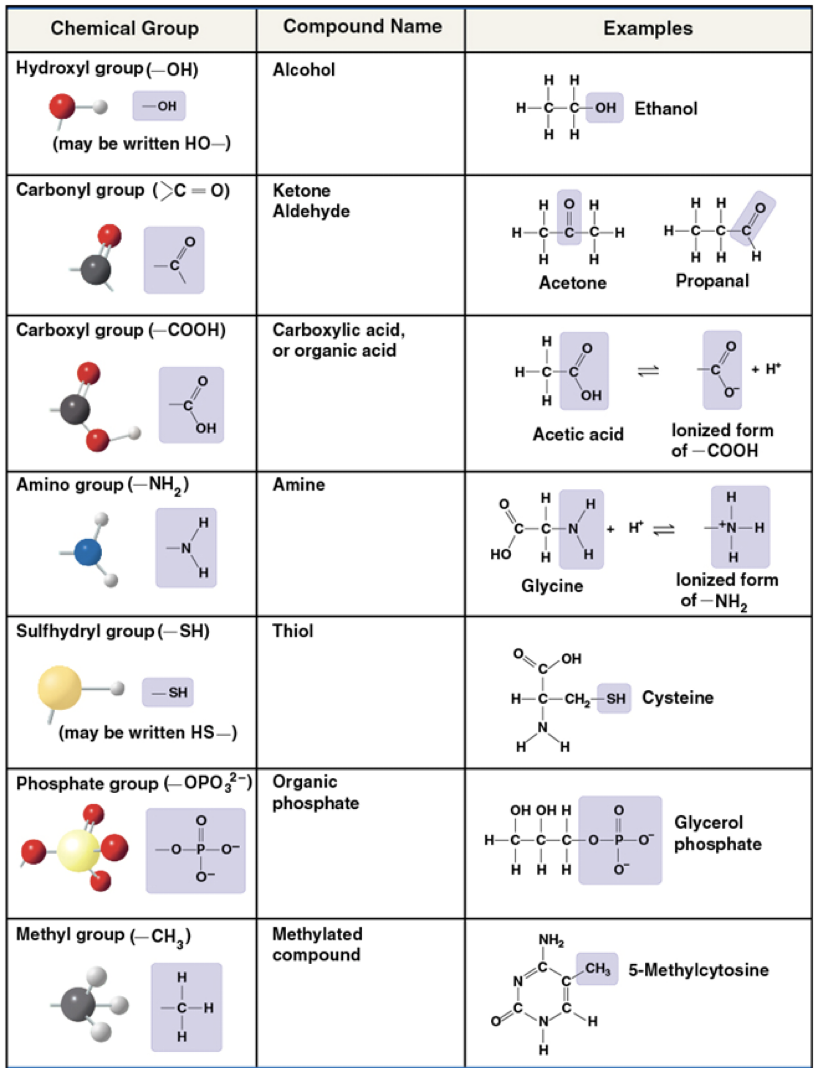
Functional groups
are the components of organic molecules that are most involved in chemical reactions
The number and arrangement of functional groups give each molecule its unique properties
Polymer
long molecule consisting of many similar building blocks
Monomer
repeating units that serve as building blocks
monomers → polymerization → polymer
What are the structures and functions of the four important classes of biological molecules?
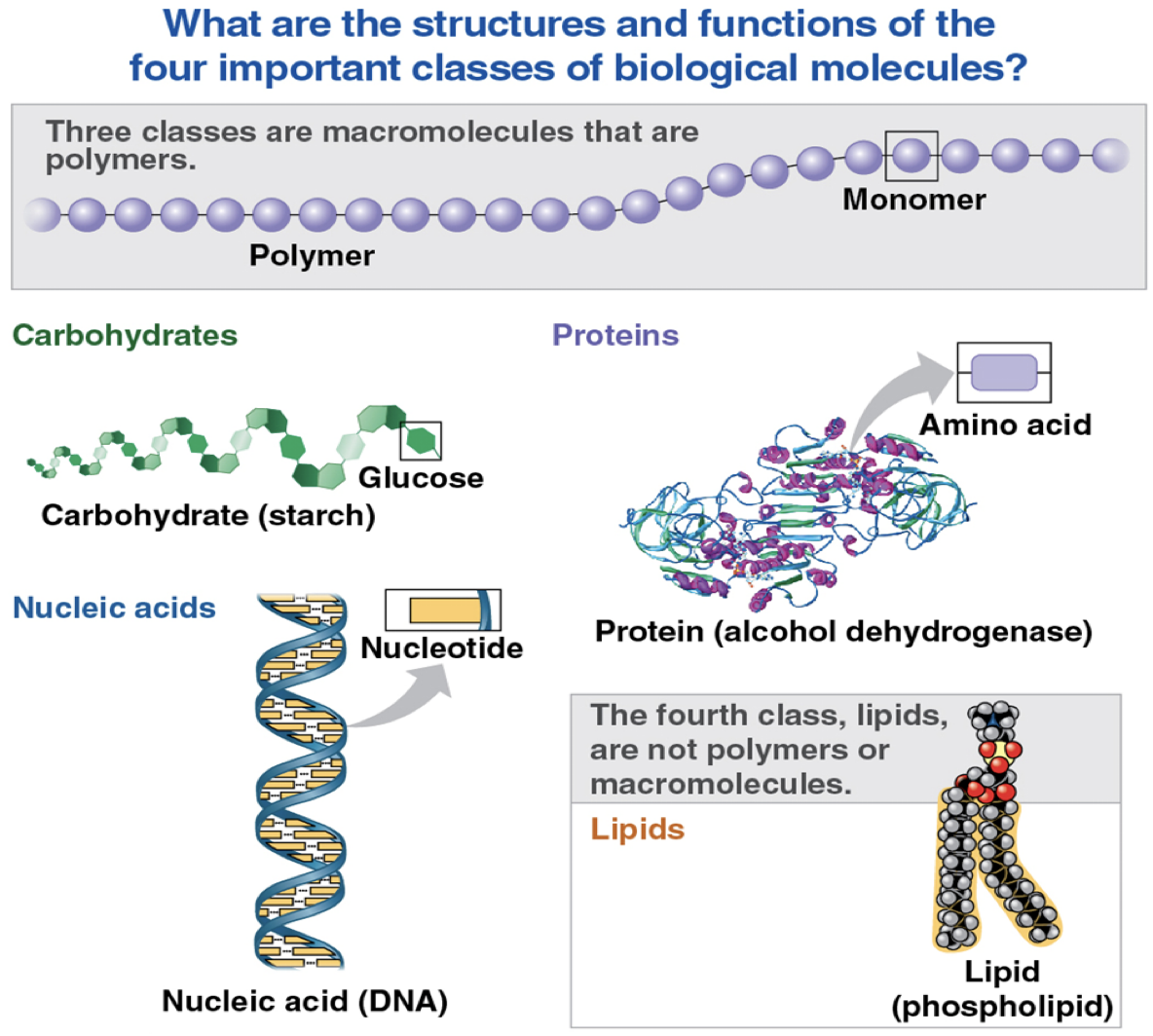
Macromolecules
large polymers—huge size
Build up & break down
Enzymes
specialized macromolecules that speed up chemical reactions
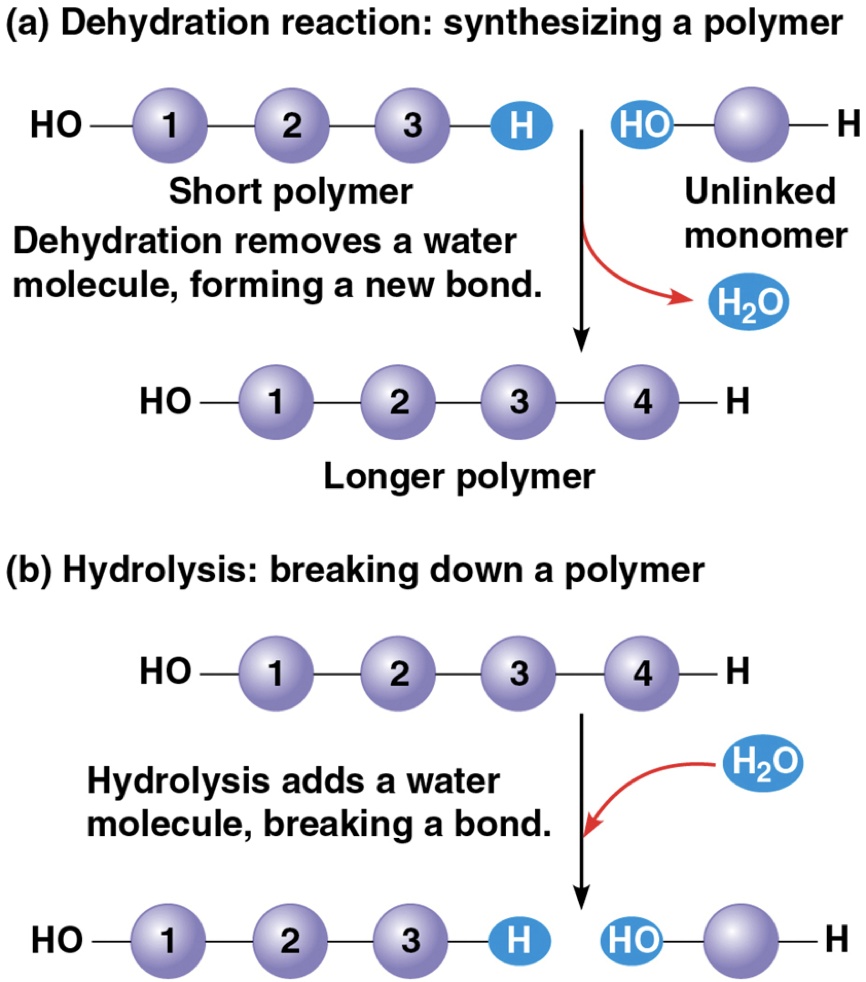
Dehydration reaction
when two monomers bond together via the loss of a water molecule
synthesizes a polymer by a removing a water molecule
forms a new bond
Hydrolysis
polymers are disassembled to monomers
breaking down a polymer through adding a water molecule & breaking a bond
essentially the reverse of the dehydration reaction
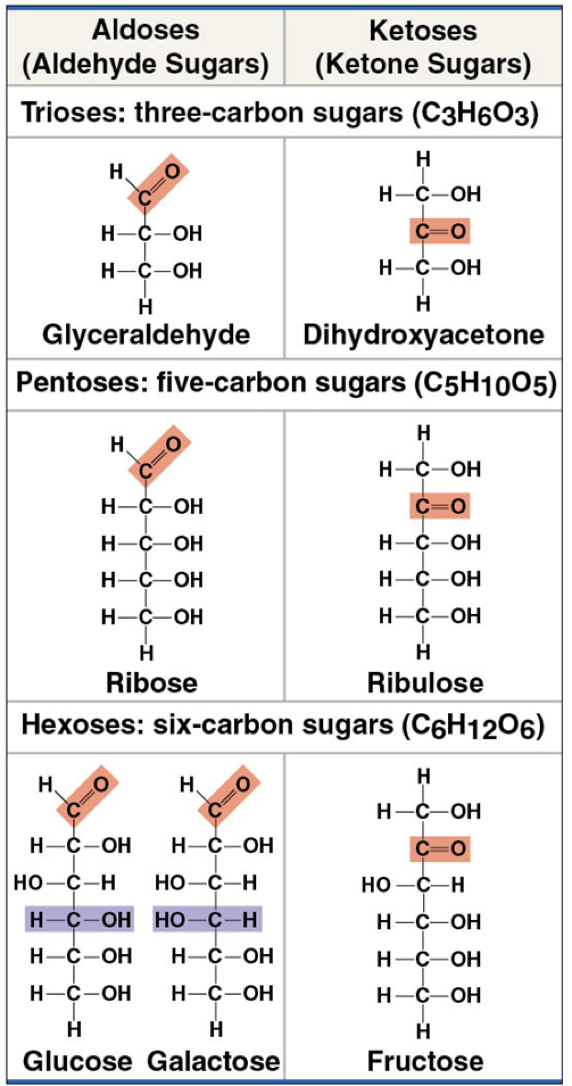
Carbohydrates
include sugars and polymers of sugars
simplest forms are monosaccharides, or simple sugars
carbohydrate macromolecules are polysaccharides
(notes) many sugars form rings (though often drawn as linear skeletons)
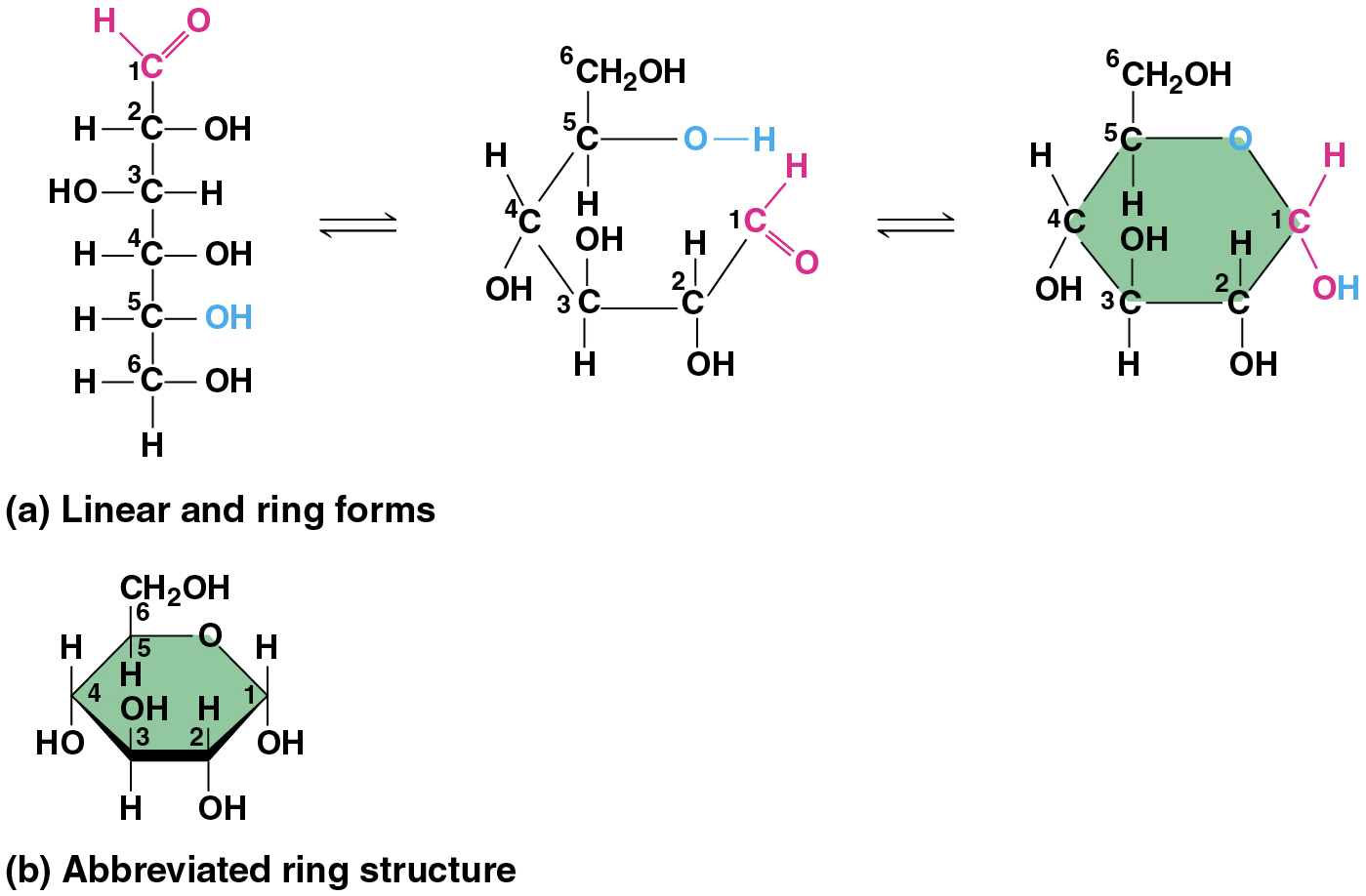
Polysaccharides
polymers composed of many sugar building blocks
have storage and structural roles
architecture and function are determined by its sugar monomers and the positions of its glycosidic linkages
Monosaccharides
have molecular formulas that are usually multiples of CH2O
Glucose (C6H12O6) is the most common monosaccharide
classified by the location of the carbonyl group (as ketose or aldose) & the number of carbons in the carbon skeleton
Disaccharide
formed when a dehydration reaction joins two monosaccharides
Glycosidic linkage
covalent bond between two monosaccharides
Starch
storage polysaccharide of plants, consists of glucose monomers
plants store surplus amounts as granules within chloroplasts and other plastics
simplest form is amylose
Glycogen
storage polysaccharide in animals
stored in liver and muscle cells
hydrolysis of this in these cells releases glucose when the demand for sugar increases
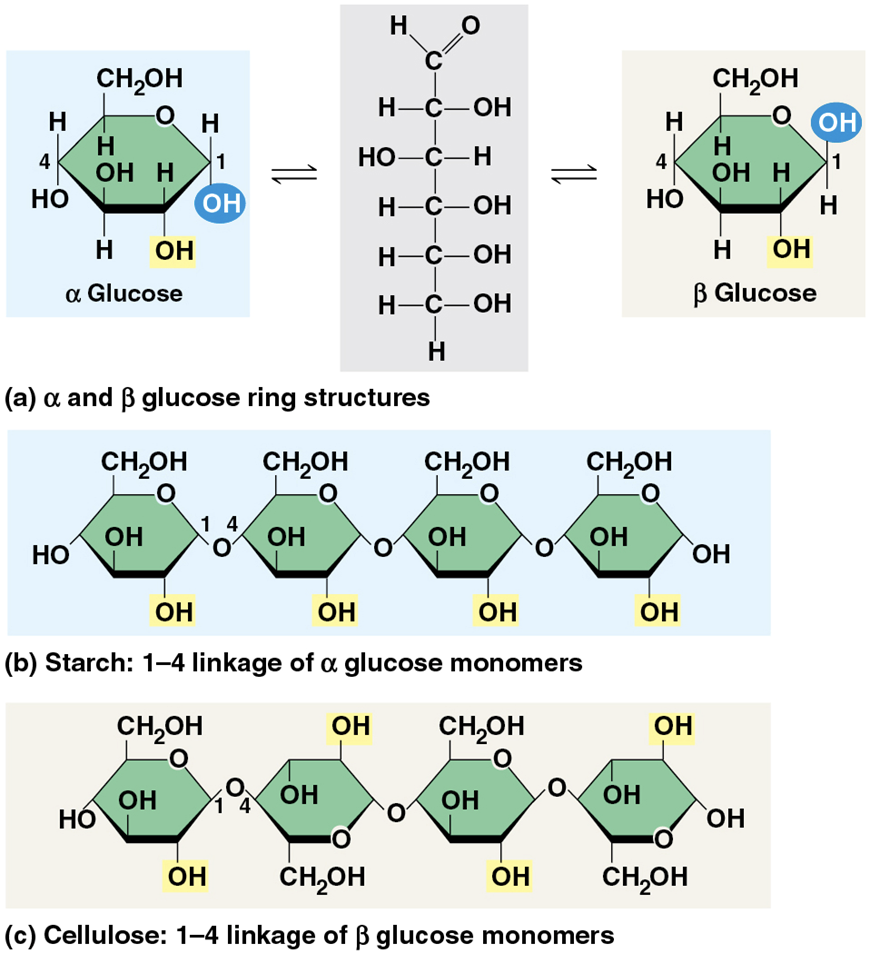
Cellulose
structural polysaccharide in the tough cell wall of plan cells
the most abundant organic polymer on Earth because it is the primary component of the cell wall of green plants, most algae, and many other organisms.
Cellulose vs Starch
Like starch, cellulose is a polymer of glucose, but the glycosidic linkages differ
The difference is based on two ring forms for glucose: alpha (α) and beta (β)
Enzymes that digest starch by hydrolyzing α linkages can’t hydrolyze β linkages in cellulose
Some hydroxyl groups on the monomers of cellulose can hydrogen-bond with hydroxyls of parallel cellulose molecules
Fats/Triglycerides
constructed from two types of smaller molecules: glycerol and fatty acids
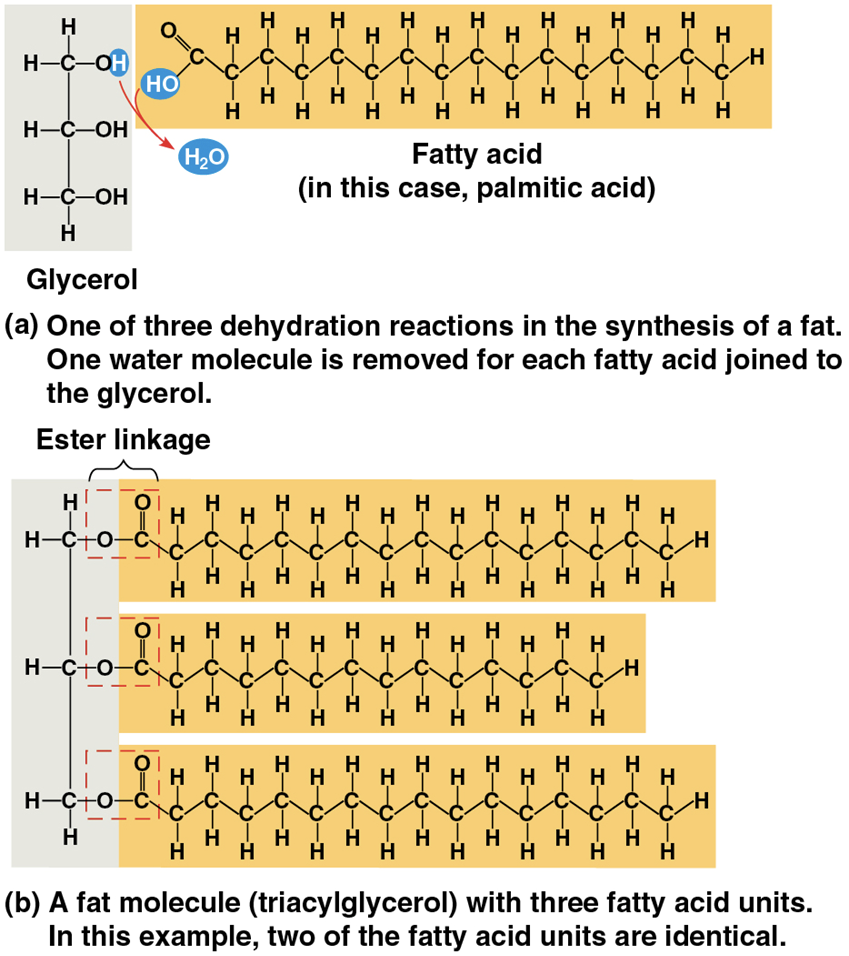
Glycerol
a three-carbon alcohol with a hydroxyl group attached to each carbon
Fatty acids
consists of a carboxyl group attached to a long carbon skeleton
Triglycerides (triacylglycerol)
three fatty acids are joined to glycerol by an ester linkage
separate from water because water molecules hydrogen-bond to each other and exclude the fats
The fatty acids in a fat can be all the same or of two or three different kinds
Major function of fats (triglycerides, not all lipids)
energy storage—long term
Adipose tissue
cushions vital organs and insulates the body
humans and other mammals store their long-term food reserves in adipose cells
Saturated fatty acids
have the maximum number of hydrogen atoms possible and no double bonds
make saturated fats
solid at room temperature
most animal fats (not all though, like omega three fatty acids in fish/seafood)
diet rich in saturated fats may contribute to cardiovascular disease, but they are still necessary in moderation
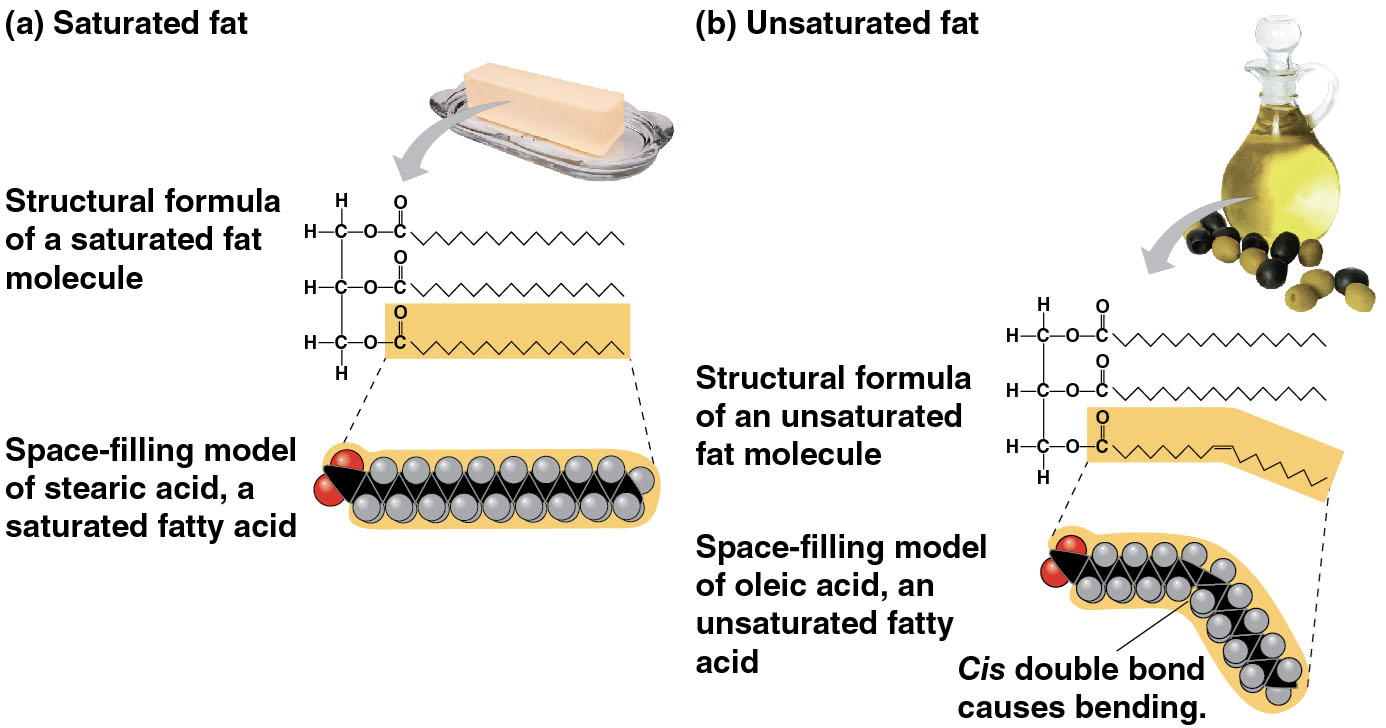
Unsaturated fatty acids
have one or more double bonds
make unsaturated fats
liquid at room temperature
usually plant fats and fish fats
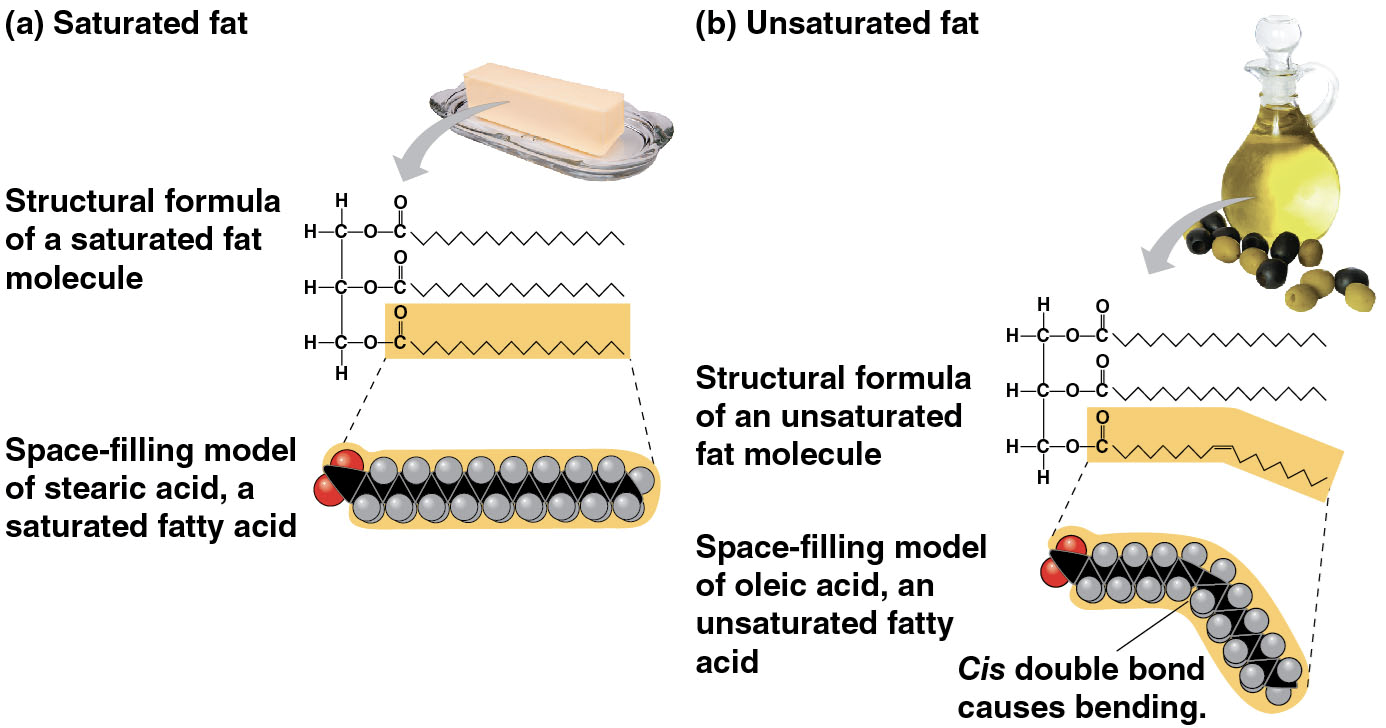
Trans fats
hydrogenation - converting unsaturated fats to saturated fats by adding hydrogen
may contribute more than saturated fats to cardiovascular disease
technically a subtype of unsaturated fat
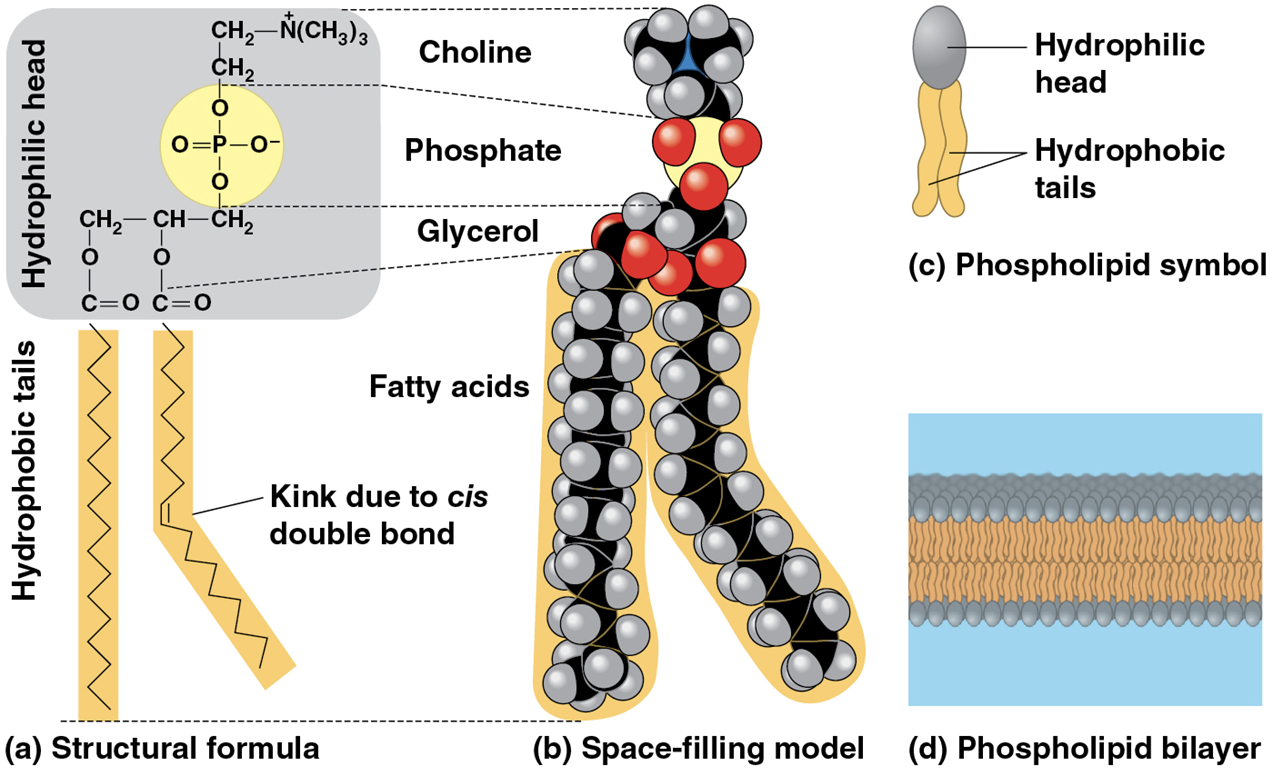
Phospholipid
two fatty acids and a phosphate group are attached to glycerol
two fatty acid tails are hydrophobic, but the phosphate group and its attachments form a hydrophilic head
(note) when added to water, they self-assemble into a bilayer, with the hydrophobic tails pointing toward interior
bilayer forms boundary between cell and external environment
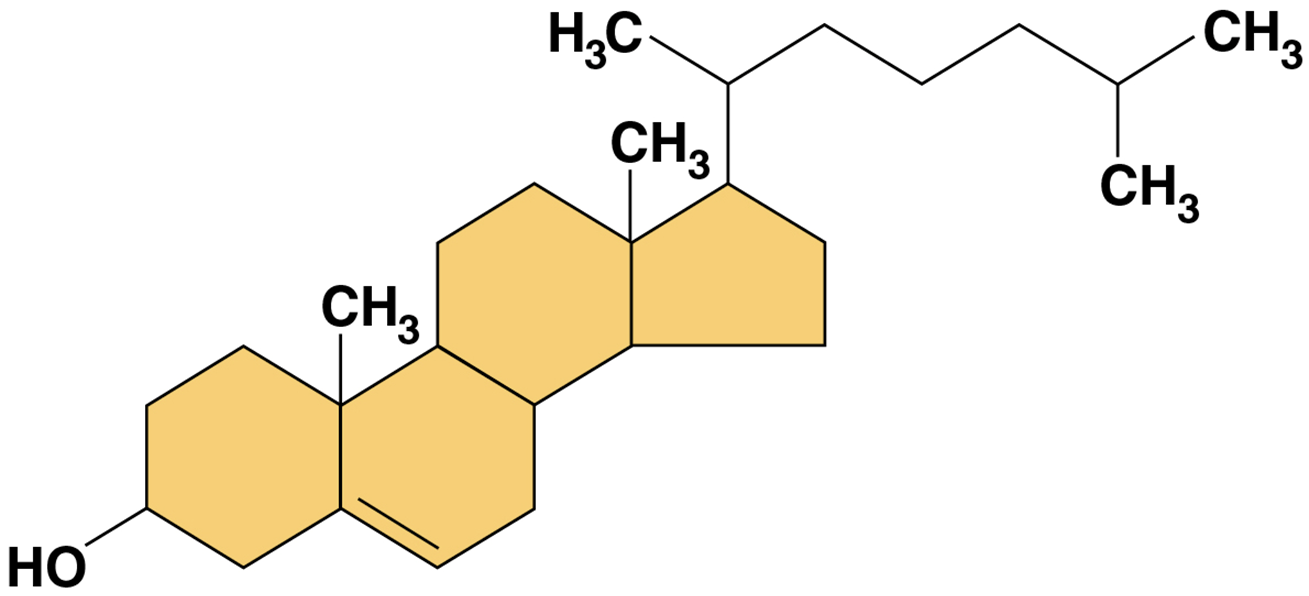
Steroids
lipids characterized by a carbon skeleton consisting of four fused rings
Cholesterol
type of steroid
a component in animal cell membranes
precursor from which other steroids are synthesized
high level in blood may contribute to cardiovascular disease
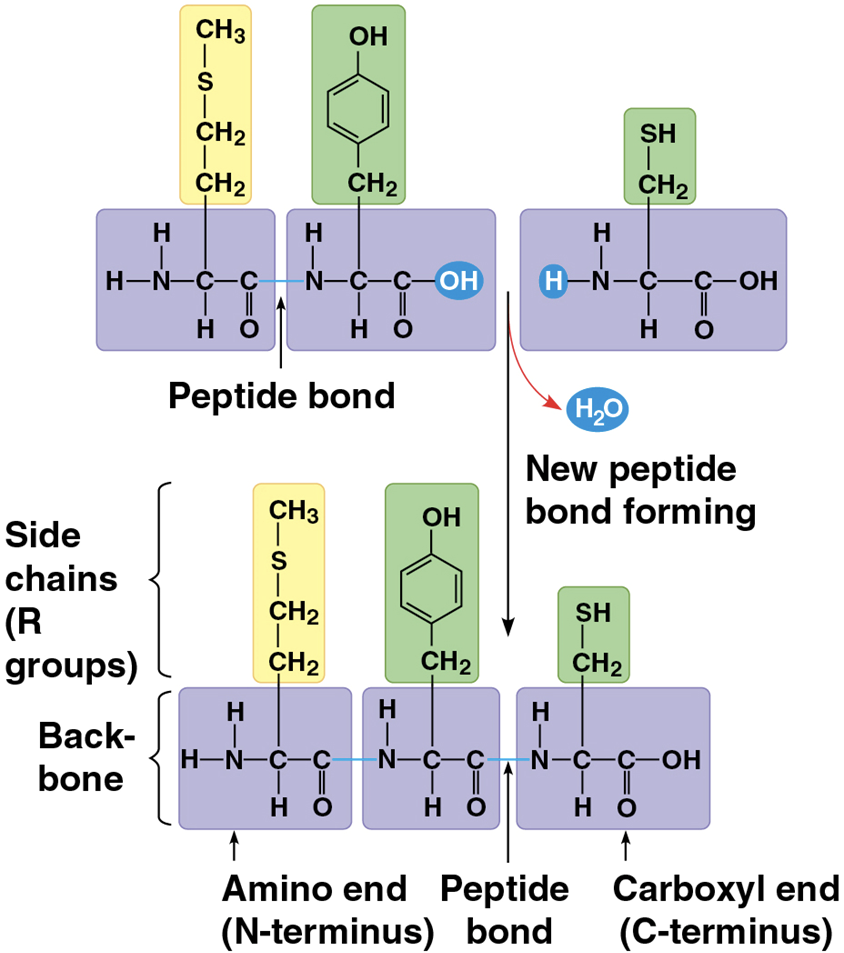
Polypeptides
unbranched polymers built from a set of 20 amino acids
Peptide bond
the covalent bond between amino acids
Protein
a biologically functional molecule that consists of one or more polypeptides
polymers with 3-dimensional shape
Enzymatic proteins
function: selective acceleration of chemical reactions
example: digestive enzymes catalyze the hydrolysis of bonds in mood molecules
Defensive proteins
function: protection against disease
example: antibodies inactive and help destroy viruses and bacteria
Storage proteins
functions: storage of amino acids
examples: Casein, the protein of milk, is the major source of amino acids for baby mammals
Transport proteins
function: transport of substances
examples: Hemoglobin, the iron-containing protein of vertebrate blood, transports oxygen from the lungs to other parts of the body
Hormonal proteins
function: coordination of an organism’s activities
example: insulin, a hormone secreted by the pancreas, causes other tissues to take up glucose, thus regulating blood sugar concentration
Receptor proteins
function: response of cell to chemical stimuli
example: receptors built into the membrane of a nerve cell detect signaling molecules released by other nerve cells
Structural proteins
function: support
example: Keratin is the protein of hair, horns, feathers, and other skin appendages.
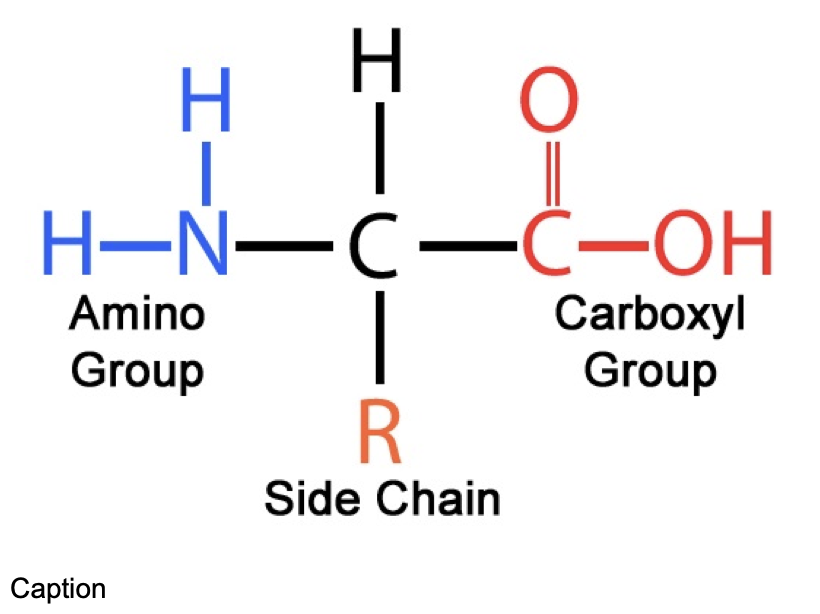
Amino acids are the monomers of proteins
central carbon atom
R side chain
carboxyl group
amino group
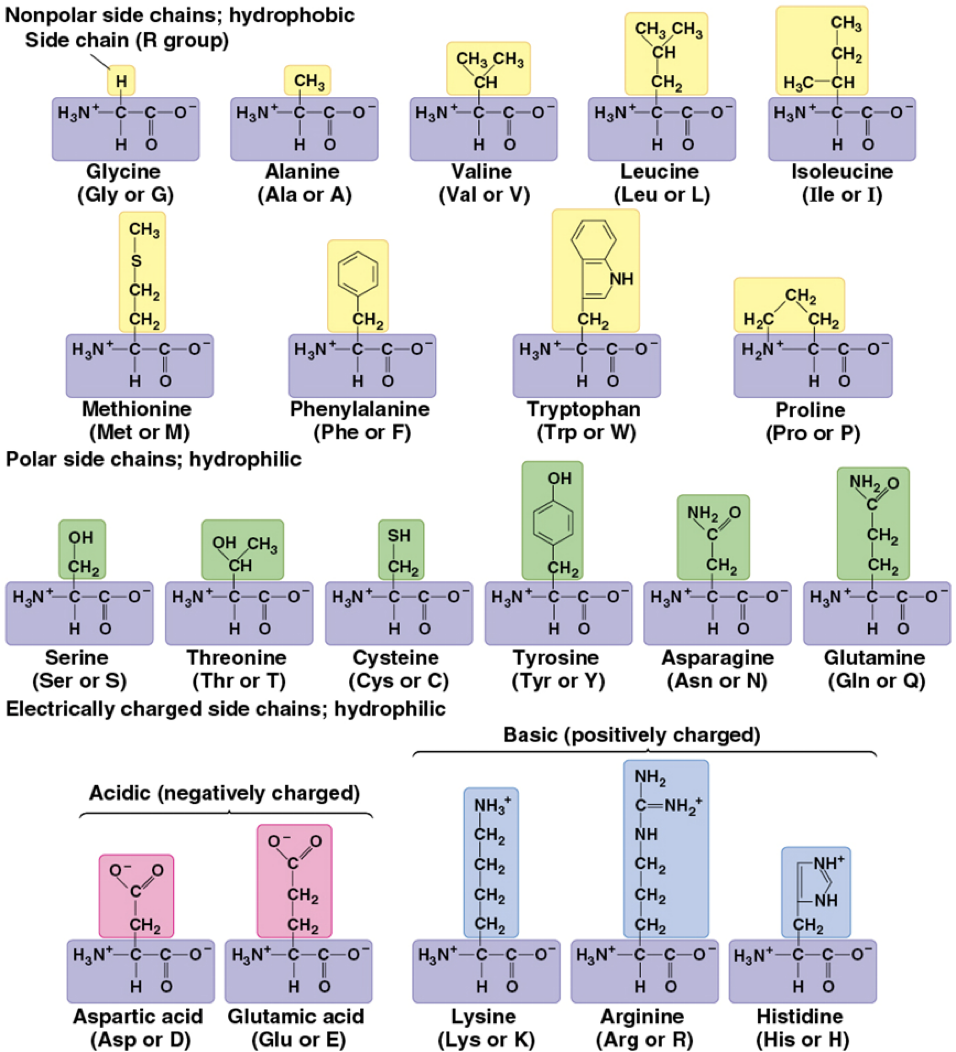
Polar vs nonpolar visually
Polar - contain atoms like oxygen or nitrogen in functional groups (e.g., -OH, -NH2, -CONH2), making them hydrophilic and able to form hydrogen bonds with water and other polar molecules
Nonpolar - consist mainly of carbon-hydrogen (CH) bonds, making them hydrophobic and tending to cluster away from water
Primary structure
a protein’s sequence of amino acids
like the order of letters in a long word
determined by inherited genetic information (genes!)
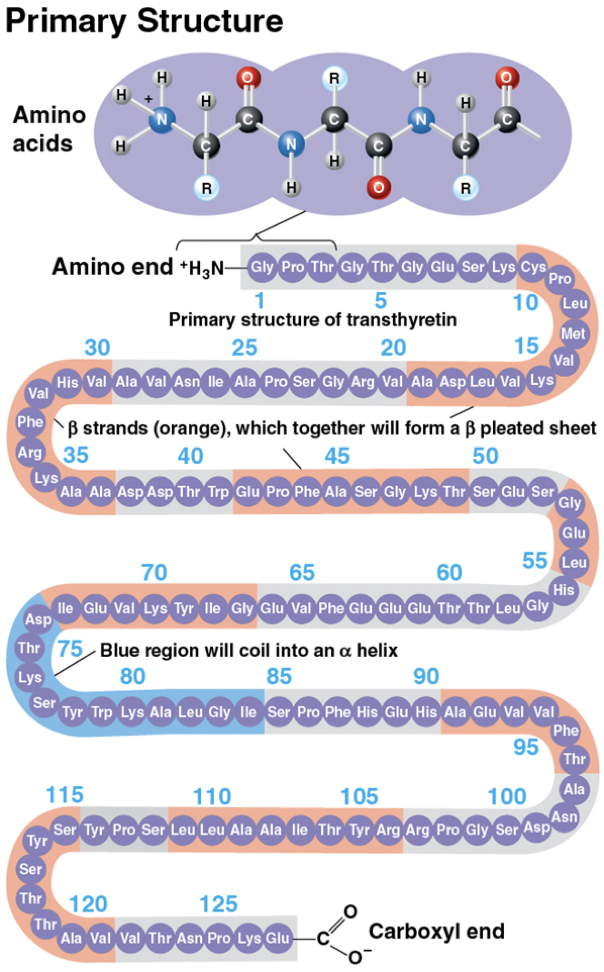
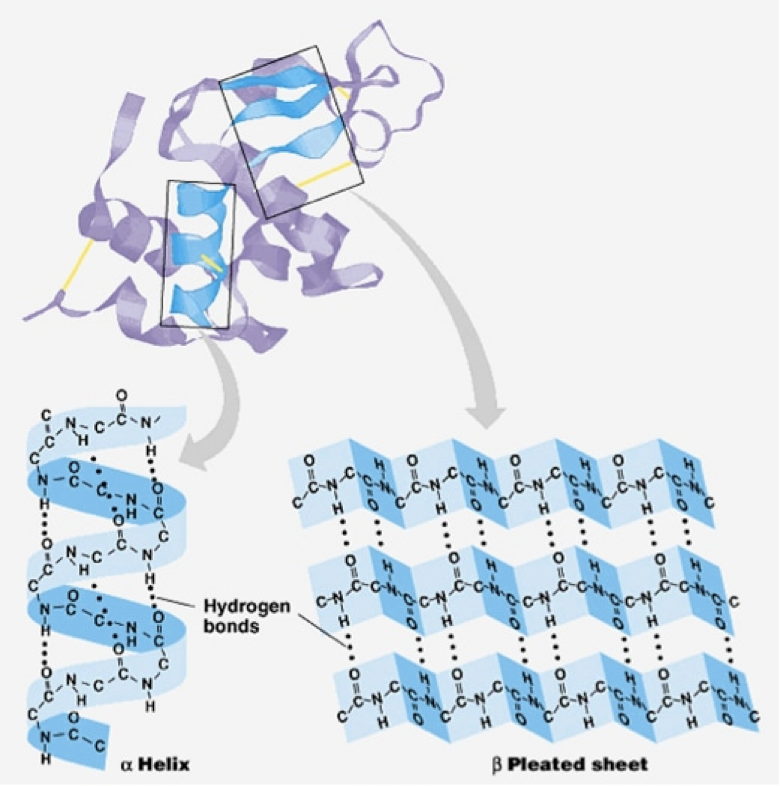
Secondary structure
coils and folds result from hydrogen bonds between repeating constituents of the polypeptide backbone (between carboxyl and amino groups)
typical structures are a coil called an α helix and a folded structure called a β pleated sheet
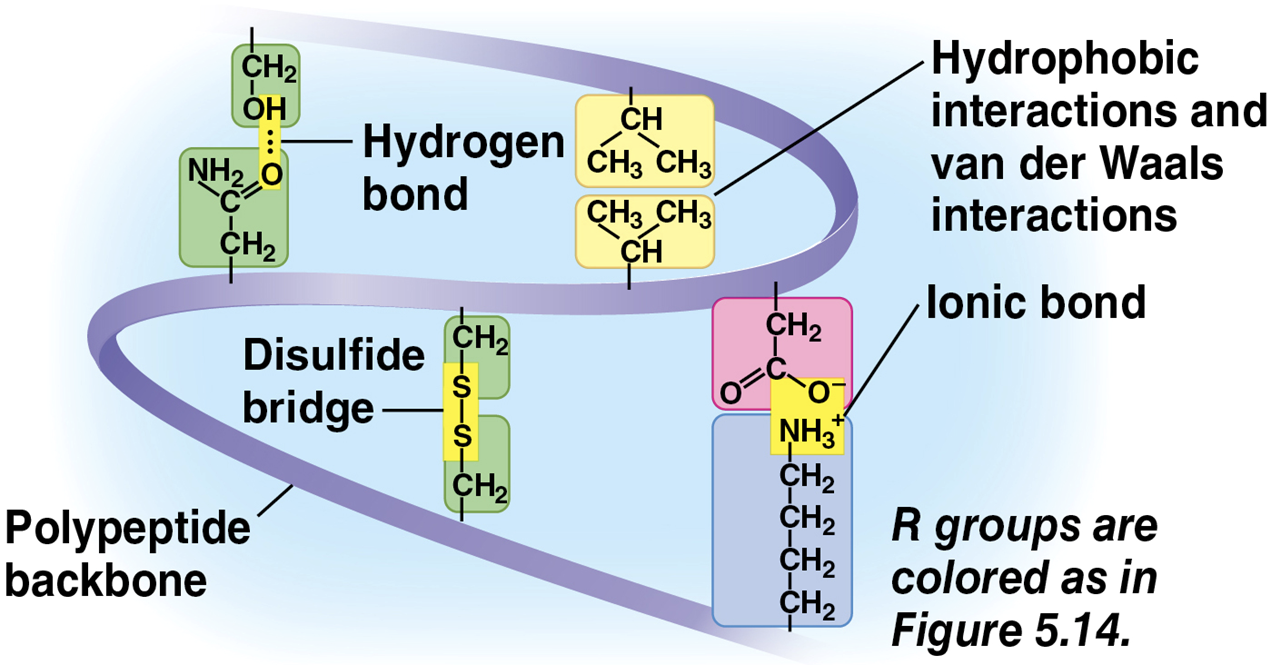
Tertiary structure
the overall shape of a polypeptide, results from interactions between R groups, rather than interactions between backbone constituents
these interactions include hydrogen bonds, ionic bonds, hydrophobic interactions, and van der Waals interactions
strong covalent bonds called disulfide bridges may reinforce the protein’s structure
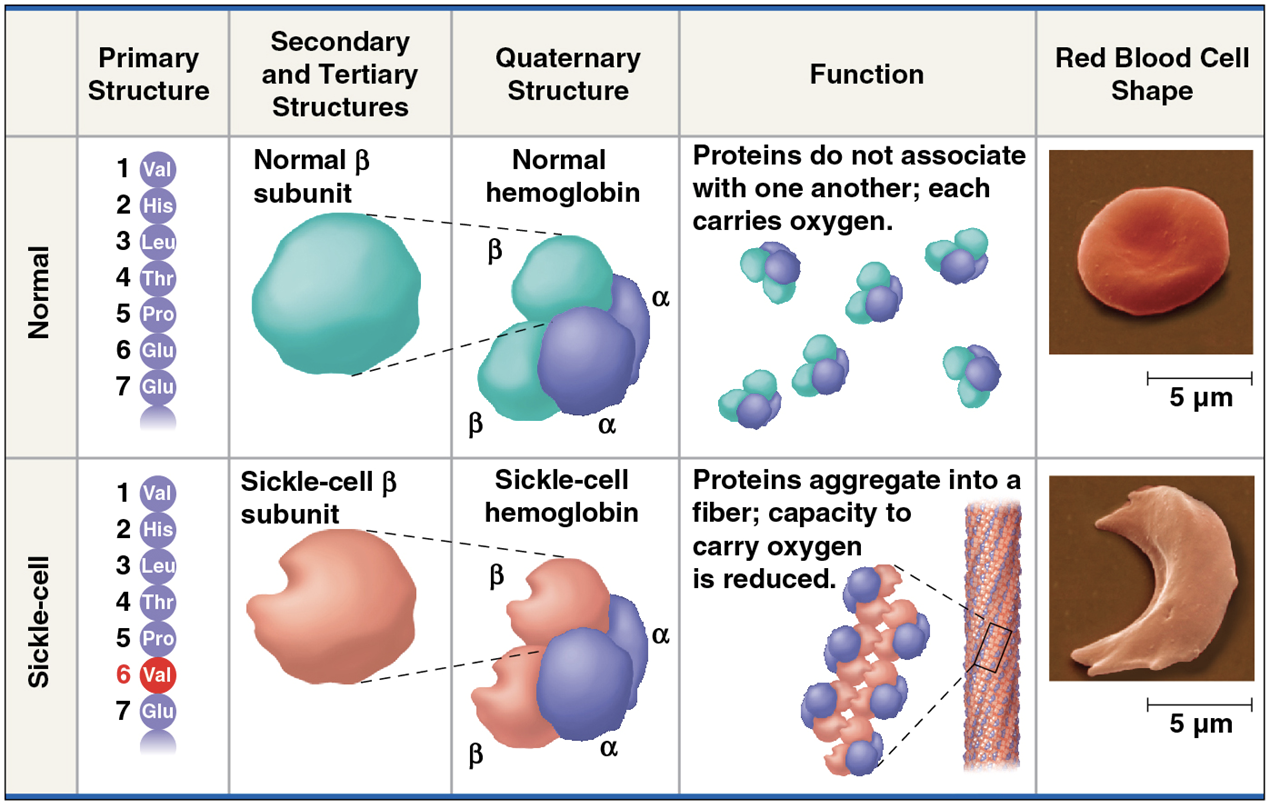
Quaternary structure
results when two or more polypeptide chains form one macromolecule
only proteins composed of two or more poly peptides can have quarternary structure
(note) usually, quaternary structures of proteins are stabilized and sub-units held together by “weak” but numerous non covalent molecular interactions, like hydrogen bonds, Van der Waals, hydrophobic and ionic interactions, as mentioned before with tertiary structures.
Structure (thus function) gone wrong
A slight change in primary structure can affect a protein’s structure and ability to function
Sickle-cell disease, an inherited blood disorder, results from a single amino acid substitution in the protein hemoglobin
The abnormal hemoglobin molecules cause the red blood cells to aggregate into chains and to deform into a sickle shape
Denaturation
loss of a protein’s native structure
denatured protein is biologically inactive
Alterations in pH, salt concentration, temperature, or other environmental factors can cause a protein to unravel
(note) ex. Extremely high fevers can be fatal: proteins in the blood tend to denature at very high body temperatures
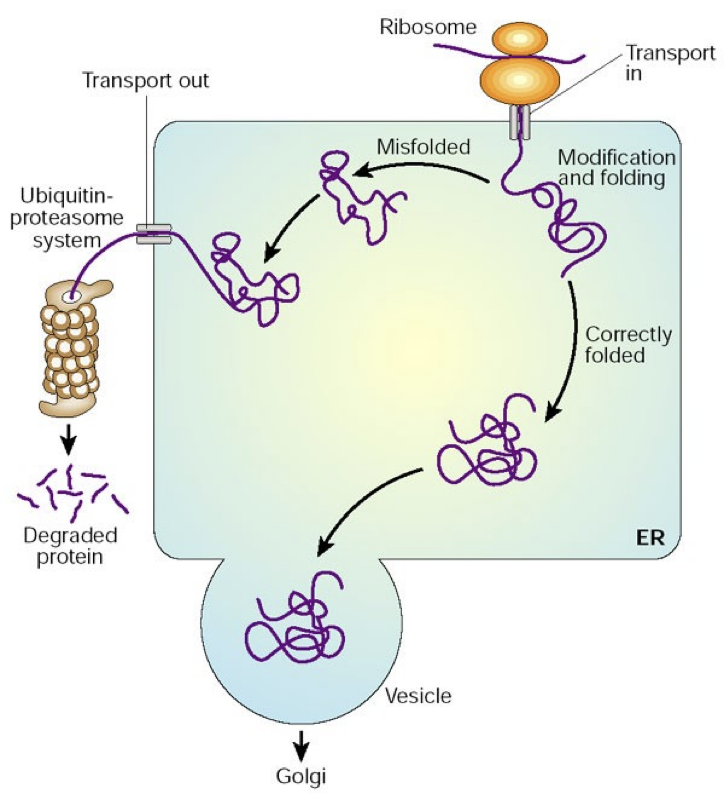
Cells fold proteins in several stages
It is hard to predict a protein’s structure from its primary structure
Most proteins probably go through several stages on their way to a stable structure
Diseases such as Alzheimer’s, Parkinson’s, and mad cow disease are associated with misfolded proteins
Gene
a unit of inheritance that programs the amino acid sequence of a polypeptide
consist of DNA
Nucleic acid
made of monomers called nucleotides
Deoxyribonucleic acid (DNA) & Ribonucleic acid (RNA)
DNA provides directions for its own replication
DNA directs synthesis of messenger RNA (mRNA) and, through mRNA, controls protein synthesis
Polynucleotides
nucleic acids are polymers called polynucleotides
made of monomers called nucleotides
Nucleotide
consists of a nitrogenous base, a pentose sugar, and one or more phosphate groups
portion of a nucleotide without the phosphate group is called a nucleoside
Nucleotide = nucleoside + phosphate group
Nucleoside = nitrogenous base + sugar
Two families of nitrogenous bases:
pyrimidines (cytosine, thymine, and uracil) have a single six-membered ring
purines (adenine and guanine) have a six-membered ring fused to a five-membered ring
ex. in DNA the sugar is deoxyribose; in RNA the sugar is ribose