Determining Organic Mechanisms, Alcohols, and Organic Oxidation-Reduction
1/45
Earn XP
Description and Tags
MCAT Prep: Organic Chemistry Part 3
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
46 Terms
Step 1
Know your Nomenclature
If given compound names in a question stem or passage, be able to draw them. If working with reaction diagrams, be able to name the compounds.
Step 2
Identify the Functional Groups
What functional groups are in the molecule? Do these functional groups act as acids or bases? How oxidized is the carbon? Are there functional groups that act as good nucleophiles, electrophiles, or leaving groups? This will help define a category of reactions that can occur with the given functional groups.
Step 3
Identify the Other Reagents
Are the other reagents acidic or basic? Are they specific to a particular reaction? Are they good nucleophiles or a specific solvent? Are they good oxidizing or reducing agents?
Step 4
Identify the Most Reactive Functional Group(s)
More oxidized carbons tend to be more reactive to both nucleophile-electrophile reactions and oxidation-reduction reactions. Note the presence of protecting groups that exist to prevent a particular functional group from reacting.
Step 5
If the reaction involves an acid or a base: protonation or deprotonation
If the reaction involves a nucleophile: nucleophile attacks electrophile, forming a bond
If the reaction involves an oxidizing or reducing agent: most oxidized functional group is oxidized or reduced, accordingly
Step 6
Consider Stereoselectivity
If there is more than one product, the major product will generally be determined by differences in strain or stability between the two molecules. Products with conjugation are significantly more stable than those without.
Conjugation
alternating single and multiple bonds
stable
Products with conjugation are significantly more ________ than those without.
higher
Alcohols have ________ boiling points than alkanes due to hydrogen bonding
acidic
weakly ________ hydroxyl hydrogen
Synthesis
addition of water to double bonds
SN1 and SN2 reactions
reduction of carboxylic acids, aldehydes, ketones, and esters
NaBH4 or LiAlH4
used to reduce aldehydes and ketones
LiAlH4
used to reduce esters and carboxylic acids
Level 0
(no bonds to heteroatoms) alkanes
Level 1
alcohols, alkyl halides, amines
Level 2
aldehydes, ketones, imines
Level 3
carboxylic acids, anhydrides, esters, amides
Level 4
(four bonds to heteroatoms) carbon dioxide
Oxidation
loss of electrons, fewer bonds to hydrogens, more bonds to heteroatoms (O, N, halogens)
Reduction
gain of electrons, more bonds to hydrogens, fewer bonds to heteroatoms
high
Good oxidizing agents have a _______ affinity for electrons or unusually ________ oxidation states
good oxidizing agents
O2, O3, Cl2, Mn7+ (permanganate), MnO4-, Cr6+, and CrO42- (chromate)
low
Good reducing agents have ________ electronegativities and ionization energies
H-
Metal hydrides are good reducing agents because they contain the ______ ion
good reducing agents
sodium, magnesium, aluminum, zinc, NaH, CaH2, LiAlH4, and NaBH4
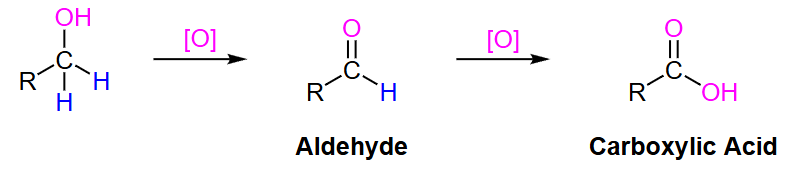
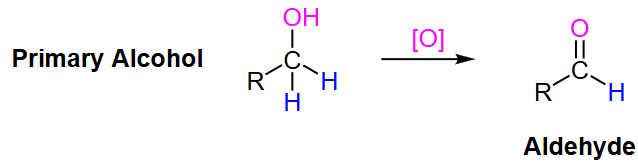
primary alcohol
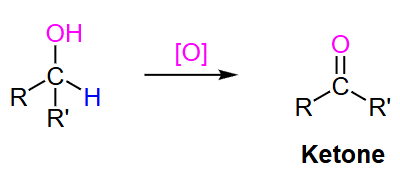
secondary alcohol
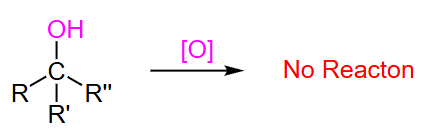
tertiary alcohol
weak
What type of oxidizing agent is needed to turn a primary alcohol to an aldehyde?
strong
What type of oxidizing agent is needed to turn an aldehyde to a carboxylic acid?
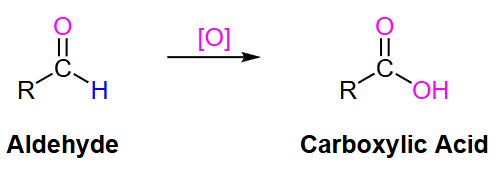
strong
What type of oxidizing agent is needed to turn a primary alcohol to a carboxylic acid?
strong or weak
What type of oxidizing agent is needed to turn a secondary alcohol to a ketone?
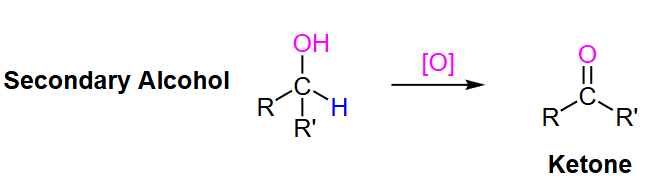
strong oxidizing agents
KMnO4
K2Cr2O7
CrO3, etc.
weak oxidizing agents
PCC
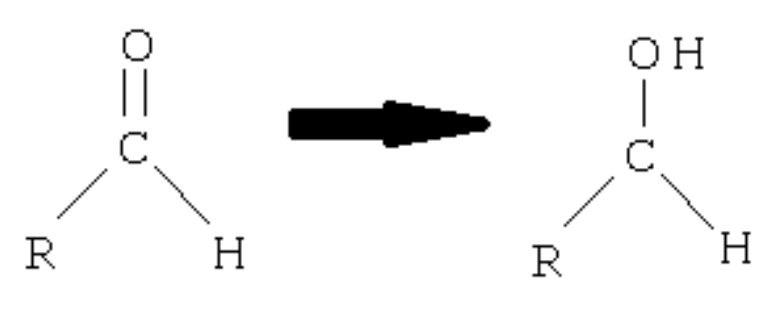
Aldehyde
? to primary alcohol
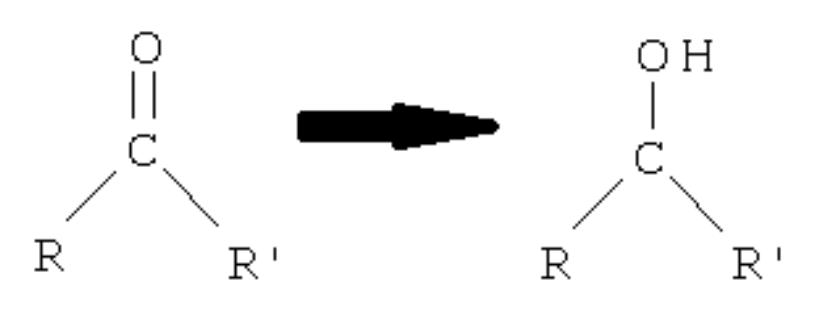
Ketone
? to secondary alcohol
Ester
? to primary alcohol + ROH
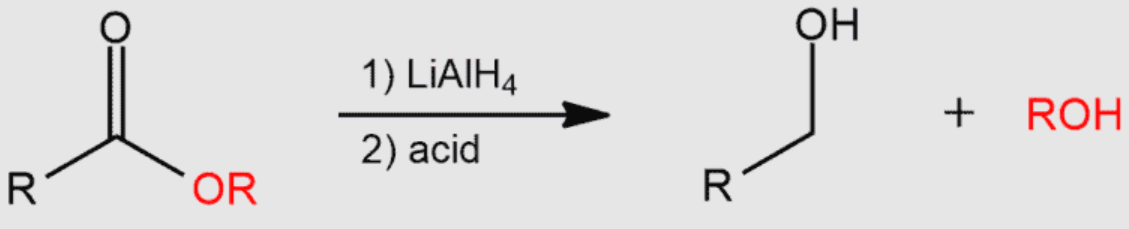
Carboxylic acid
? to primary alcohol + H2O
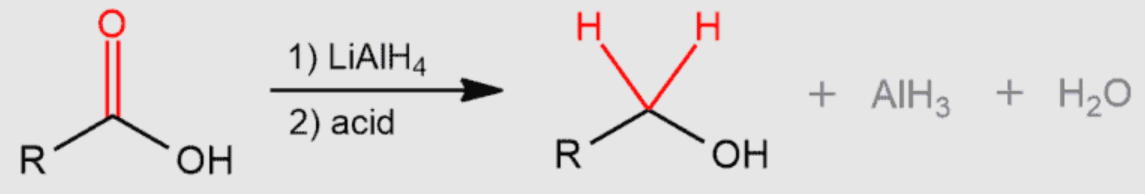
weak or strong
What type of reducing agent is needed to turn aldehydes to primary alcohols and ketones to secondary alcohols?
strong
What type of reducing agent is needed to turn esters and carboxylic acids into primary alcohols?
weak reducing agent
NaBH4
strong reducing agent
LiAlH4
mesylates; tosylates
Alcohols can be converted to _________ or _________ to make them better leaving groups for nucleophilic substitution reactions.
Mesylates
are derived from methanesulfonic acid (-SO3CH3)
Tosylates
are derived from toluenesulfonic acid (-SO3C6H4CH3)
protecting groups
Alcohols can be used as ____________ for carbonyls, as reaction with a dialcohol forms an unreactive acetal.