C6- Chem OCR Gateway-1
1/22
Earn XP
Description and Tags
Part 1
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
Alkanes are a group of saturated…
hydrocarbons. Saturated means that they only have single carbon bonds, so no double bonds.
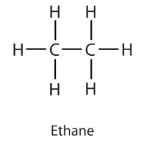
How do I find the general formula for alkanes?
Look at the first few alkanes (methane, ethane, propane)
Count Carbons (C) and Hydrogens (H) in each.
Notice every time you add one carbon, you add two hydrogens.
So write it as CnH2n+2 (n represents number or carbons)
What do alkanes look like?
They are colourless compounds, which gradually change as the number of carbon atoms increase.
How do alkanes react?
Alkanes are unreactive, but can combust (burn) and be cracked (broken) into smaller molecules.
Is methane an alkane?
Yes, methane is an alkane and is a
Alkanes are hydrocarbons…
meaning that only have hydrogen and carbon.
What happens to hydrocarbons in the presence of air?
They undergo combustion (burning). This reaction always ends with the products water and carbon dioxide.
What is Methane’s combustion reaction?
CH4 + 2O2 → CO2 + 2H2O
Methane + Oxygen (air) → Carbon Dioxide and Water
When does incomplete combustion happen?
When there is a limited supply of oxygen. This produces carbon dioxide and water.
Why don’t car engines burn gasoline properly?
Engines are not 100% efficient, so some unburnt hydrocarbons like carbon monoxide and soot are released, which causes pollution.
Carbon dioxide from combustion (burning) is a problem because…
it contributes to global warming.
What are alkenes?
Alkenes are unsaturated hydrocarbons, which contain at least one C=C double bond. The double bond makes alkenes reactive.
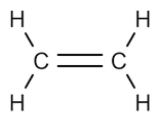
Give the molecular formula for the first four alkenes
Ethene: C2H4
Propene: C3H6
Butene: C4H8
Pentene: C5H10
What does unsaturated mean?
It means the hydrocarbon has a double bond (C=C) that can open and form more single bonds with atoms.
How do alkanes differ from alkenes in terms of reactivity?
Alkenes are more reactive because their double bond can open and react ; alkanes only have single bonds and are less reactive.
What does the number in but-1-ene or pent-2-ene mean?
It’’s where the double bond starts, number from the end closest to the bond
What products form in the complete combustion of alkenes?
Carbon Dioxide and Water
Why do alkenes tend to undergo incomplete combustion?
They have a higher carbon to hydrogen ratio, leading to smoky flames and soot.
Write the complete combustion of butene (C4H8)
C4H8 + 6O2 → 4CO2 + 4H2O
What happens in the addition reaction of alkenes?
The double vond C=C breaks open, and becomes a single bond, as new atoms add to the carbon bond.
How do you turn an alkene into an alkane (hydrogenation) ?
Add hydrogen (H2) to the double bond, using nickel at 150 degrees as a catalyst - so the double bond becomes single.
What happens when alkenes react with halogens (halogenation)?
Halogen atoms attach themselves to each carbon atom of the double bond.
How does bromine water tell alkenes and alkanes apart?
Alkenes will go from orange to colourless, as bromine reacts with the double bond.
Alkanes will stay orange, as there is no reaciton with a single bond.