IB Biology- B1.1: Carbohydrates and Lipids
1/74
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
75 Terms
Carbohydrates
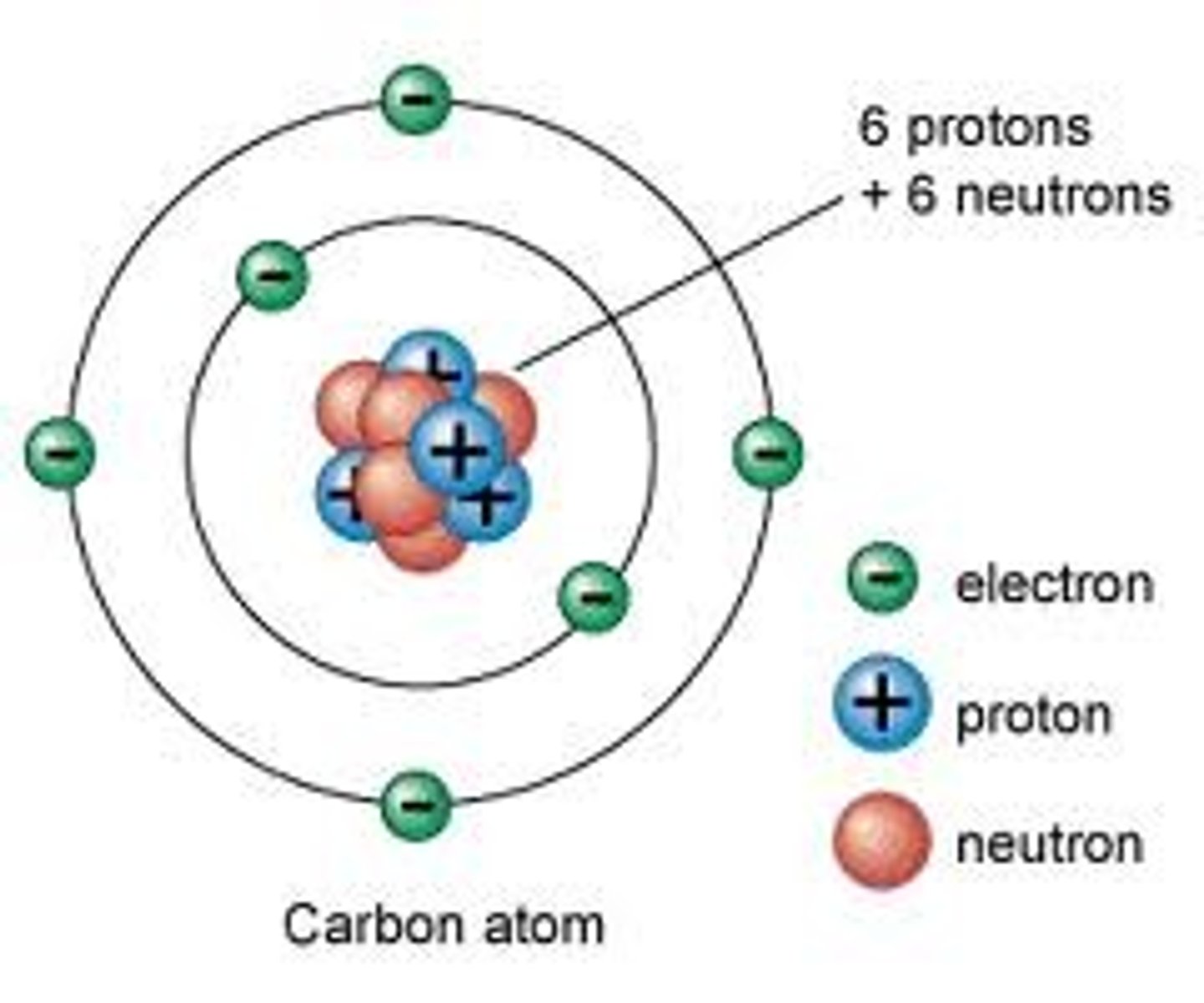
What is the electronic configuration of carbon and what does this mean?
Carbon atoms have the electron structure 2,4 (6 electrons) meaning they have 4 electrons in their outer shell therefore can make single bonds with 4 other atoms.
This bonding brings stability to the molecule formed as it takes a large amount of energy to break the covalent bonds.
What do the prefixes kilo, centi, milli, micro, and nano mean?
Kilo: x 10^3
Centi: x 10^-2
Milli: x 10^-3
Micro: x 10^-6
Nano: x 10^-9
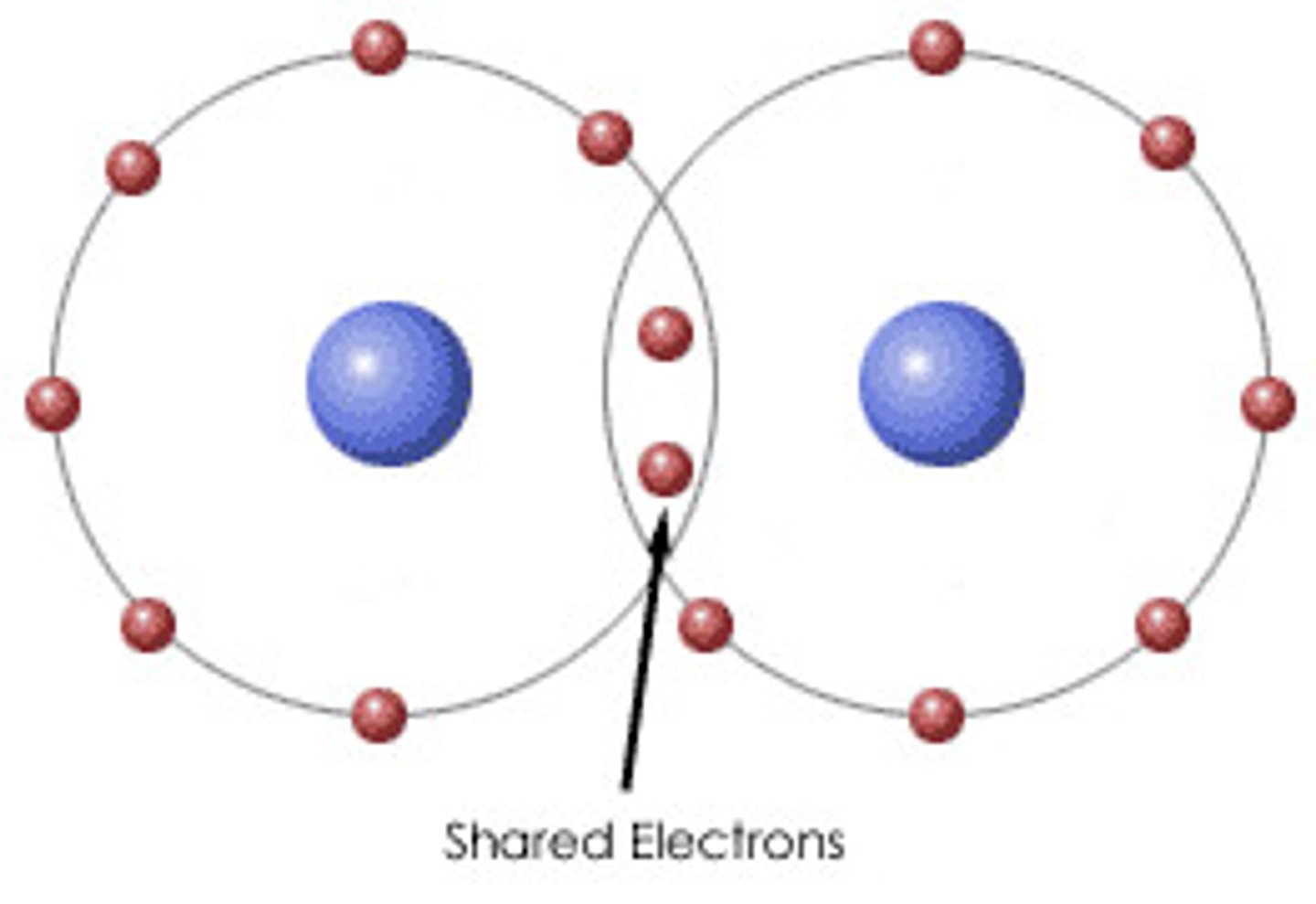
What is a covalent bond?
A strong bond formed as a result of the electrostatic force of attraction between a shared pair of electrons and the nuclei of the atoms.
What type of bonds can carbons make?
- Single (saturated)
- Double (unsaturated)
- Triple
What types of structures can carbon atoms form?
- Straight chain carbons
- Ring structures
What is the term for the formation of polymers?
Polymerisation
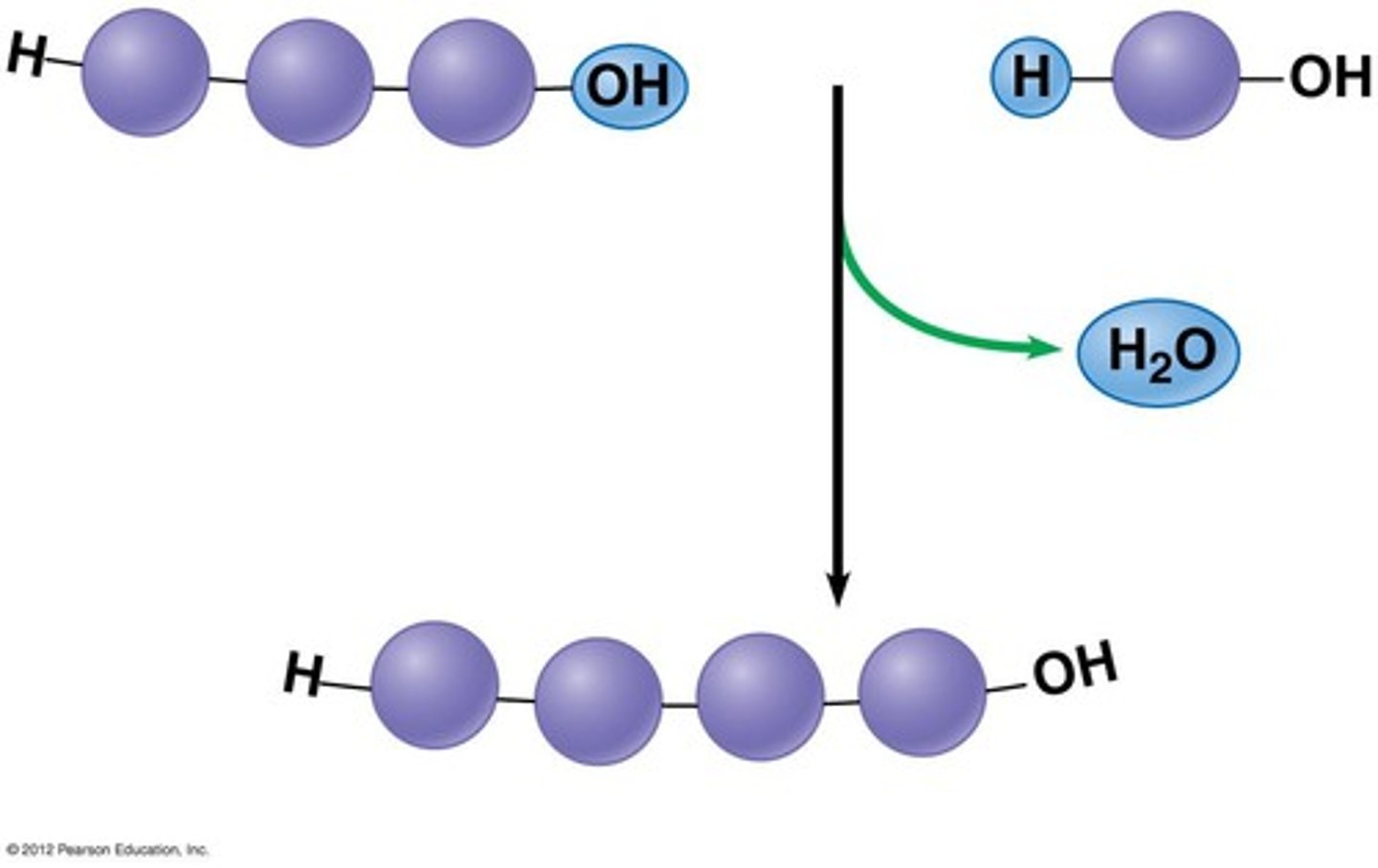
What is a condensation reaction?
When two molecules join together and a bond forms between them. A water molecule is also evolved.
What can condensation reaction be used for in the body?
Building glycogen from glucose in animal cells or starch from glucose in plant cells
What is a hydrolysis reaction?
The addition of a water molecule to a polymer, breaking the bond between subunits.
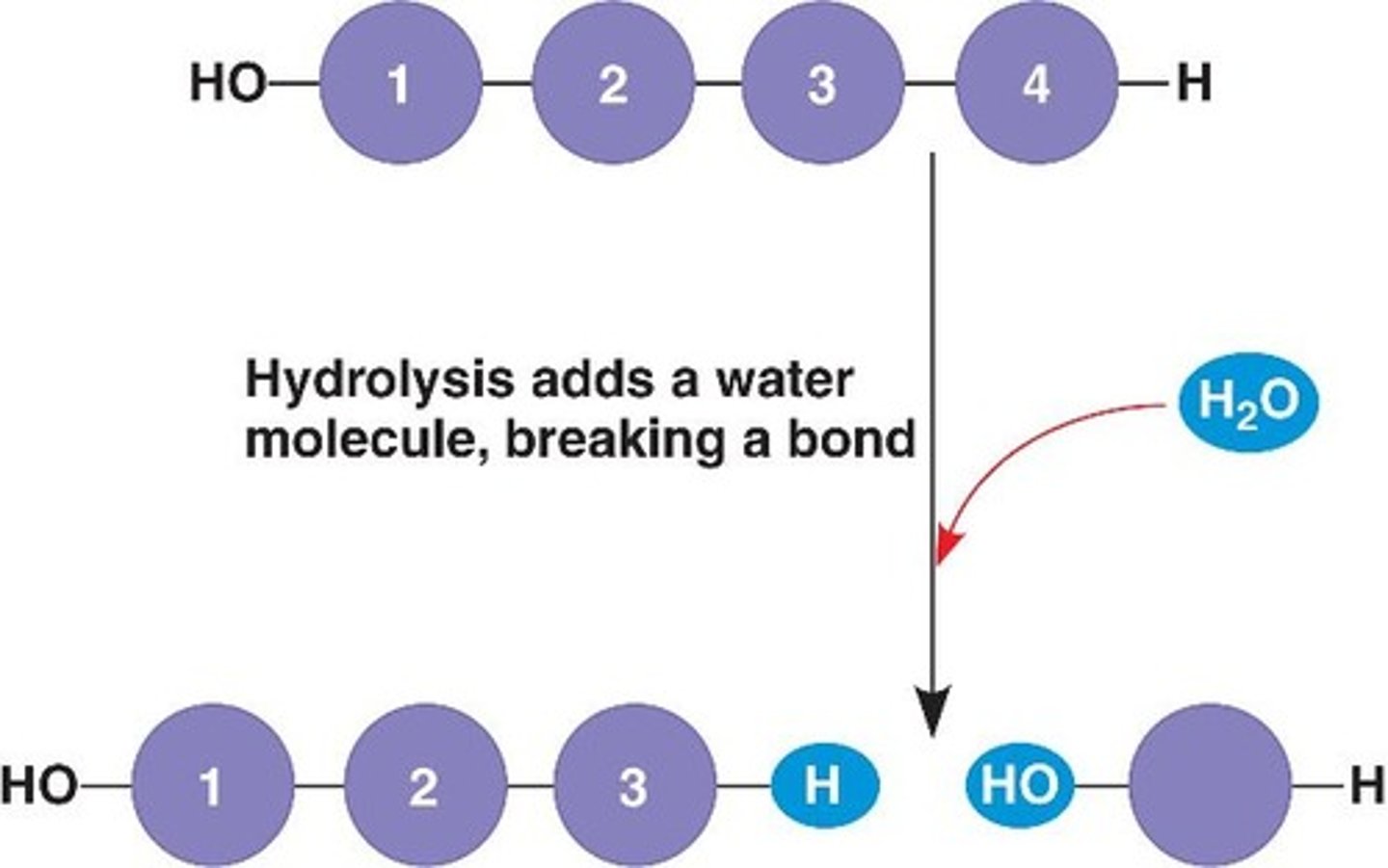
Why is it called a hydrolysis reaction?
The water molecules split to provide the -H and -OH groups that are incorportated to produce monomers.
What can hydrolysis be used for in the body?
- Breaking down glycogen/starch into glucose.
- Digestion where digestive enzymes hydrolise polymers into monomers
What are carbohydrates?
Organic molecules which contain Carbon, Hydrogen, and Oxygen in a 2:1 ratio (H:C,O)
Why are carbohydrates important?
They are needed in even the simplest cells (bacteria) for important processes such as respiration.
What type of molecule is a carbohydrate?
A macromolecule (big molecule) made up of many small repeating units (these are called polymers).
What are the monomers of carbohydrates?
Monosaccharides e.g. glucose
What is a hexose sugar?
A monosaccharide with six carbon atoms in each molecule (forming a hexagonal shape)
What is the chemical formula of glucose?
C6H12O6
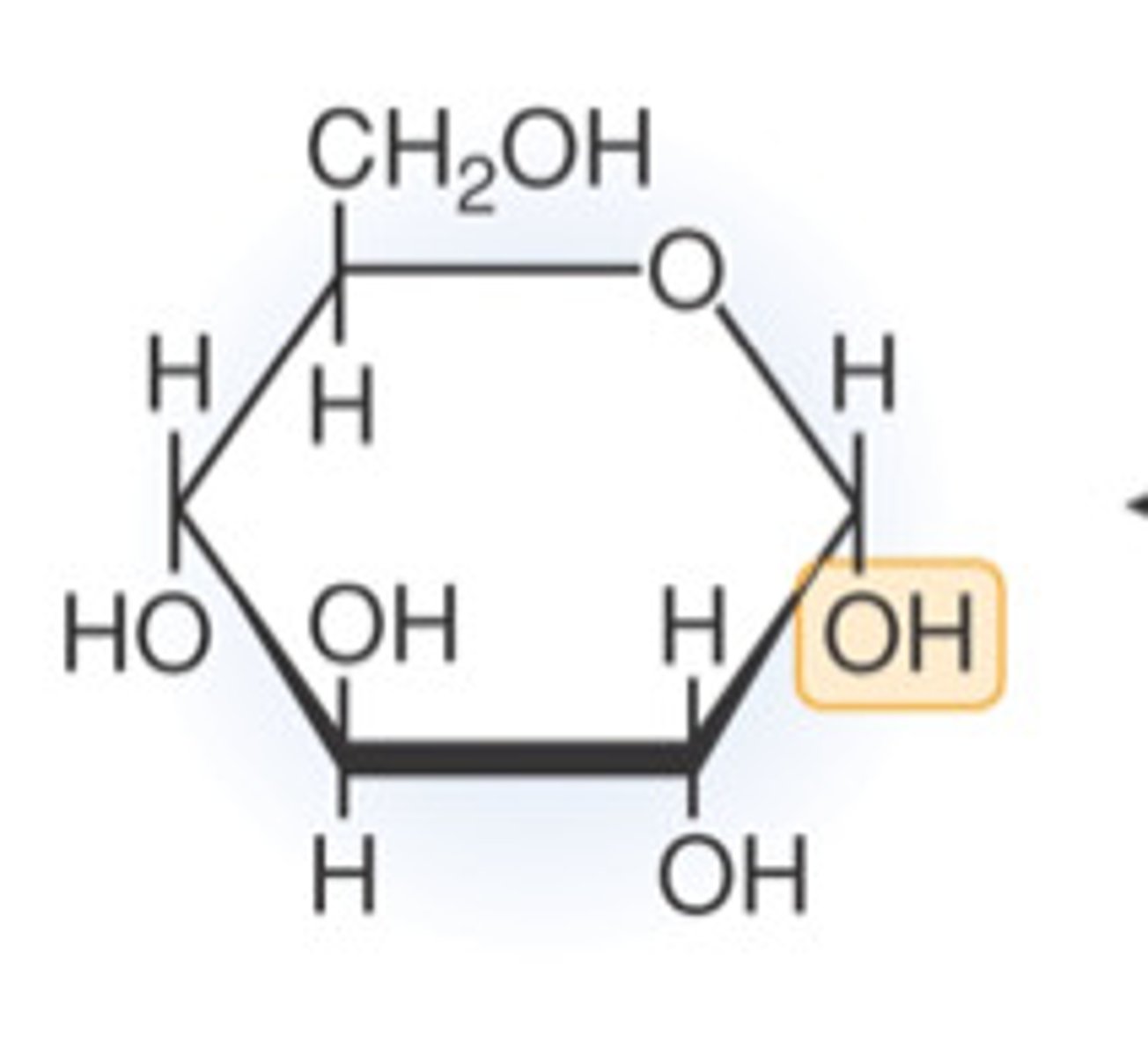
What is the structure of alpha glucose?
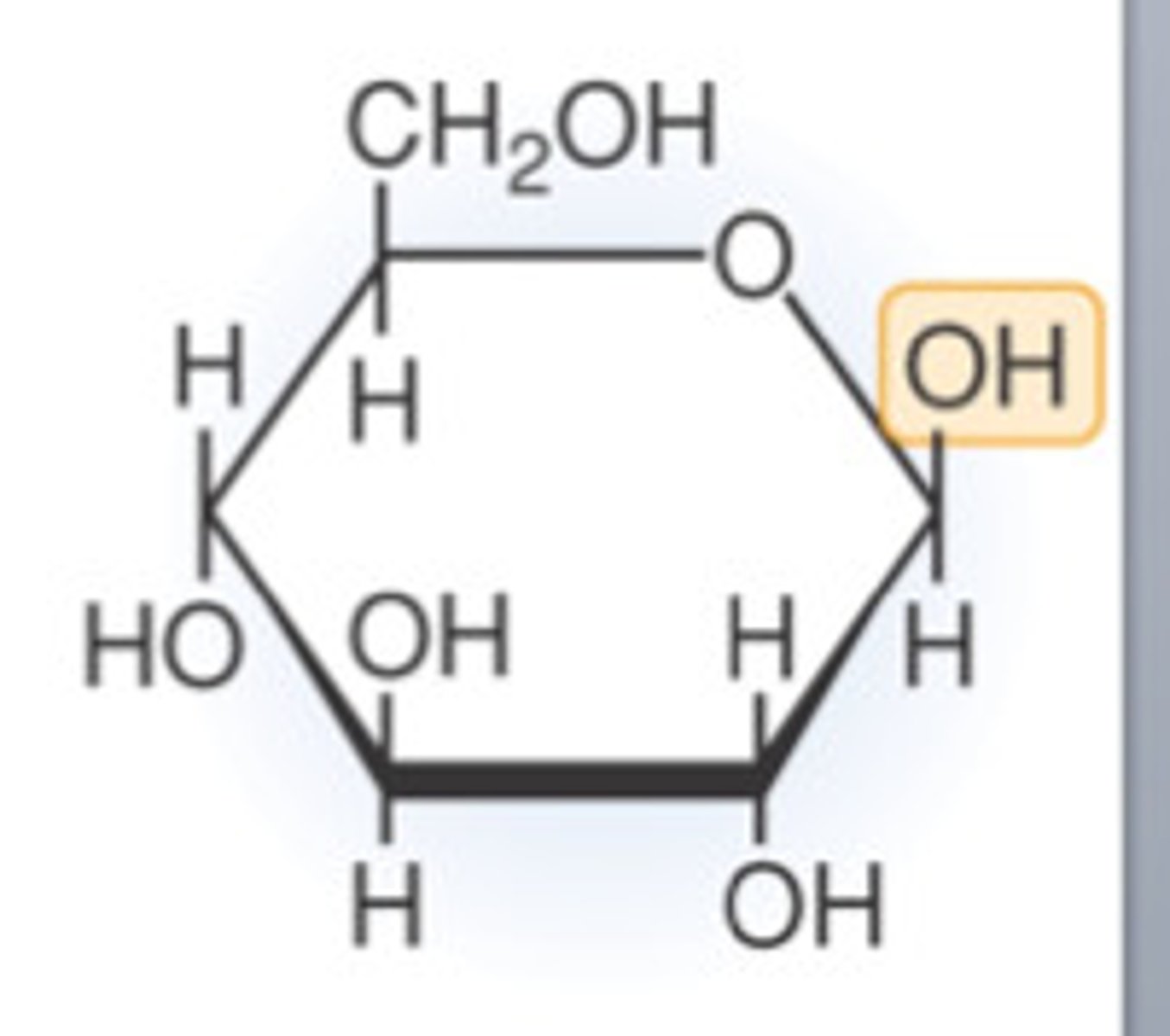
What is the structure of beta glucose?
What is a pentose sugar?
A monosaccharide with 5 carbon atoms in each molecule (forming a pentagonal shape)
What is the chemical formula of ribose?
C5H10O5
What is the structure of ribose?
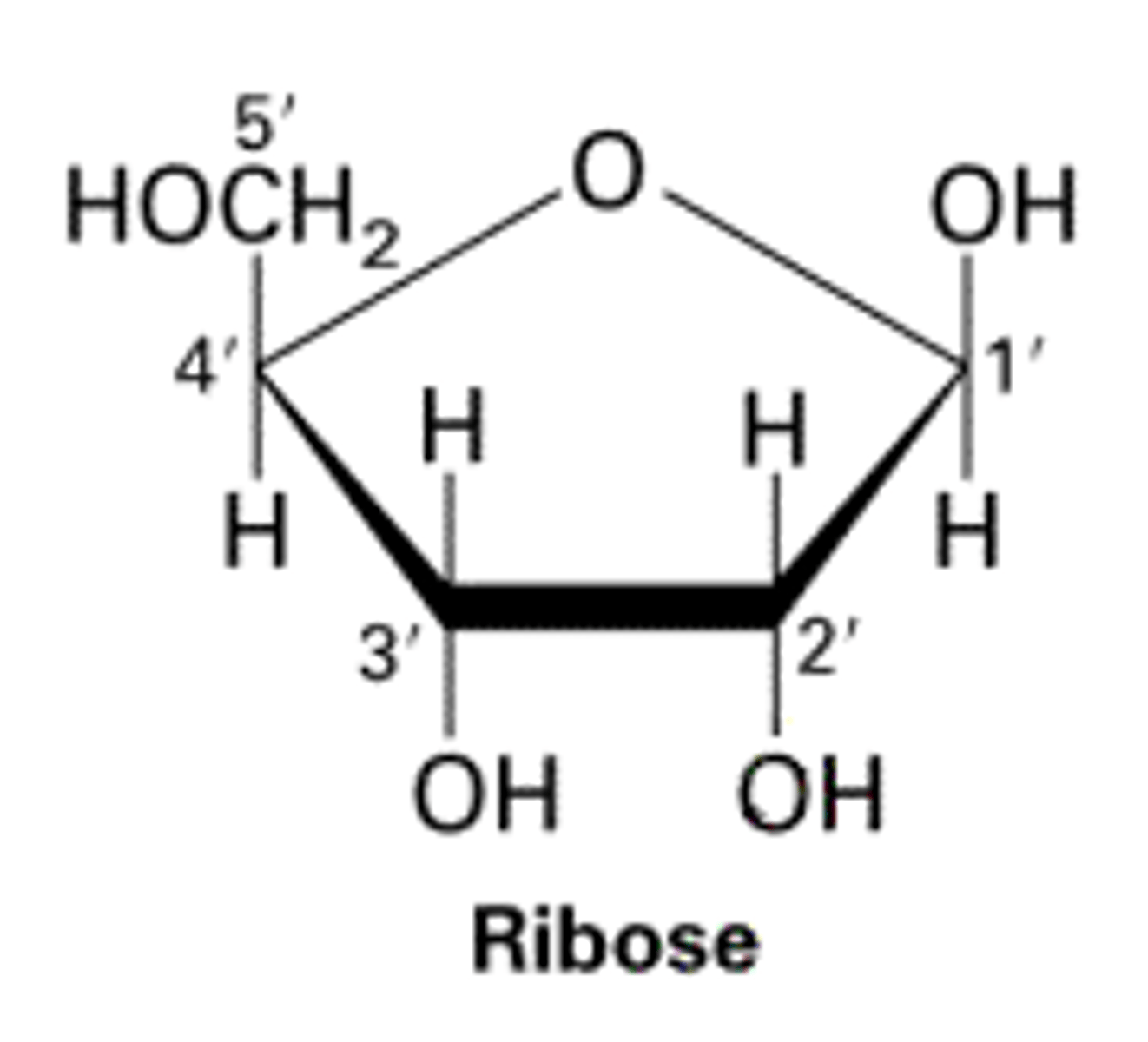
What is a disaccharide?
A pair of monosaccharides produced through a condensation reaction
What bond forms in a disaccharide?
Glycosidic bond ((1,4) carbons are joined by an oxygen)
Which disaccharide is formed by joining two glucose molecules?
Maltose
What is the chemical formula of maltose?
C12H22O11
What does maltose look like?
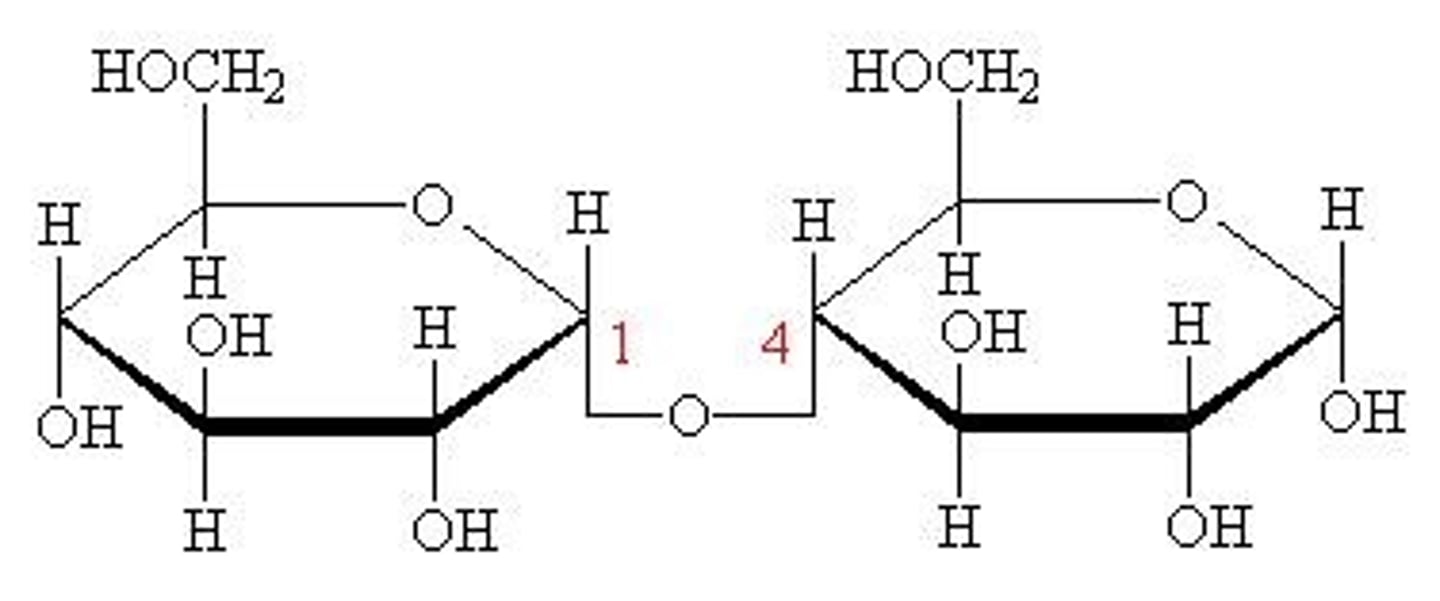
What is anabolism?
The process of constructing molecules from monomers. It involves a condensation reaction.
E.g. During photosynthesis plants produce glucose then store it as starch
What is catabolism?
The breaking down of complex molecules into simple molecules. It involves a hydrolysis reaction.
E.g. Digestion (breaking down starch into maltose then glucose), respiration (oxidation of glucose which involves breaking down the covalent bonds to release energy
What is metabolism?
The total of all the chemical reactions taking place in a living organism
What are the properties of glucose?
- Solubility
- Transportability
- Chemical stability
- Energy yield from oxidation
Why are these properties of glucose?
- High solubility comes as a result of glucose being a polar molecule (due to its OH groups) therefore readily dissolves in a polar solvent (water)
- Transportability as a result of its solubility meaning it can easily circulate in blood and the fluids between cells
- Chemical stability due to it being joined together with dtrong covalent bonds that don't break easily
- Energy yield from oxidation as a result of releasing lots of chemical energy when its covalent bonds are broken (therefore it is a good energy store)
What are 3 important polysaccharides that glucose forms and what are they used for?
- Glycogen (animal storage carbohydrate found in the cytoplasm)
- Cellulose (used for building cell walls)
- Starch (plant storage carbohydrate found in the chloroplasts)
Where are there many glycogen granules?
In muscle (for respiration) and liver cells (because they carry out so many metabolic reactions)
Why are storage molecules important?
They are insoluble and so prevent osmosis and cell bursting
What is a structure/function relationship?
A structure function relationship is where the structure of an organelle/molecule/cells is directly related to its function e.g. Enzymes, red blood cells.
What is the structure of starch and how does it relate to its function?
Starch is made up of straight chains of alpha glucose monosaccharides wound into a tight helix to form amylose and branching amylopectin (branch by connecting to C6 instead of C4).
- The compact helical shape of amylose means many glucose molecules can be packed tighty and stored in a small space.
- It is insoluble due to its large size so doesn't affect the osmotic potential of the cell and doesn't diffuse out of the cell
- It easily hydrolyses to form alpha glucose molecules which are used in respiration.
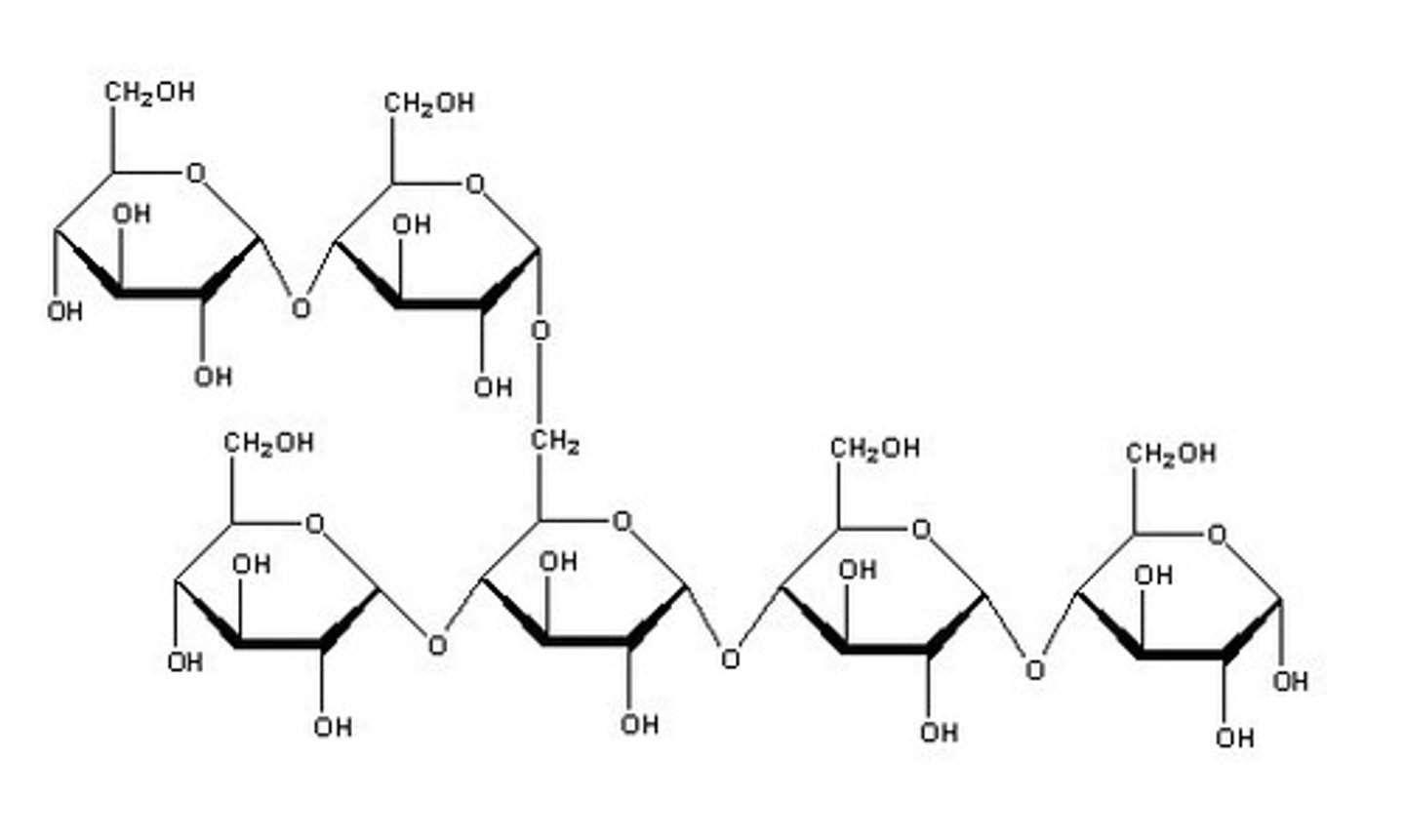
What is the structure of glycogen and how does it relate to its function?
Glycogen is similar to Amylopectin but made up of shorter branching chains of alpha glucose molecules and is more highly branched (1,6 glycosidic bonds).
- The branching glycogen is a good storage molecule as the number of branches and ends of the molecule mean glucose can be released very quickly compared to straight chains with only 2 ends.
- This shape is also more compact which is essential for storage.
- Glycogen is also insoluble so doesn't diffuse out or affect the osmotic potential of the cell.
- It is hydrolysed to form alpha glucose molecules which are used in respiration.
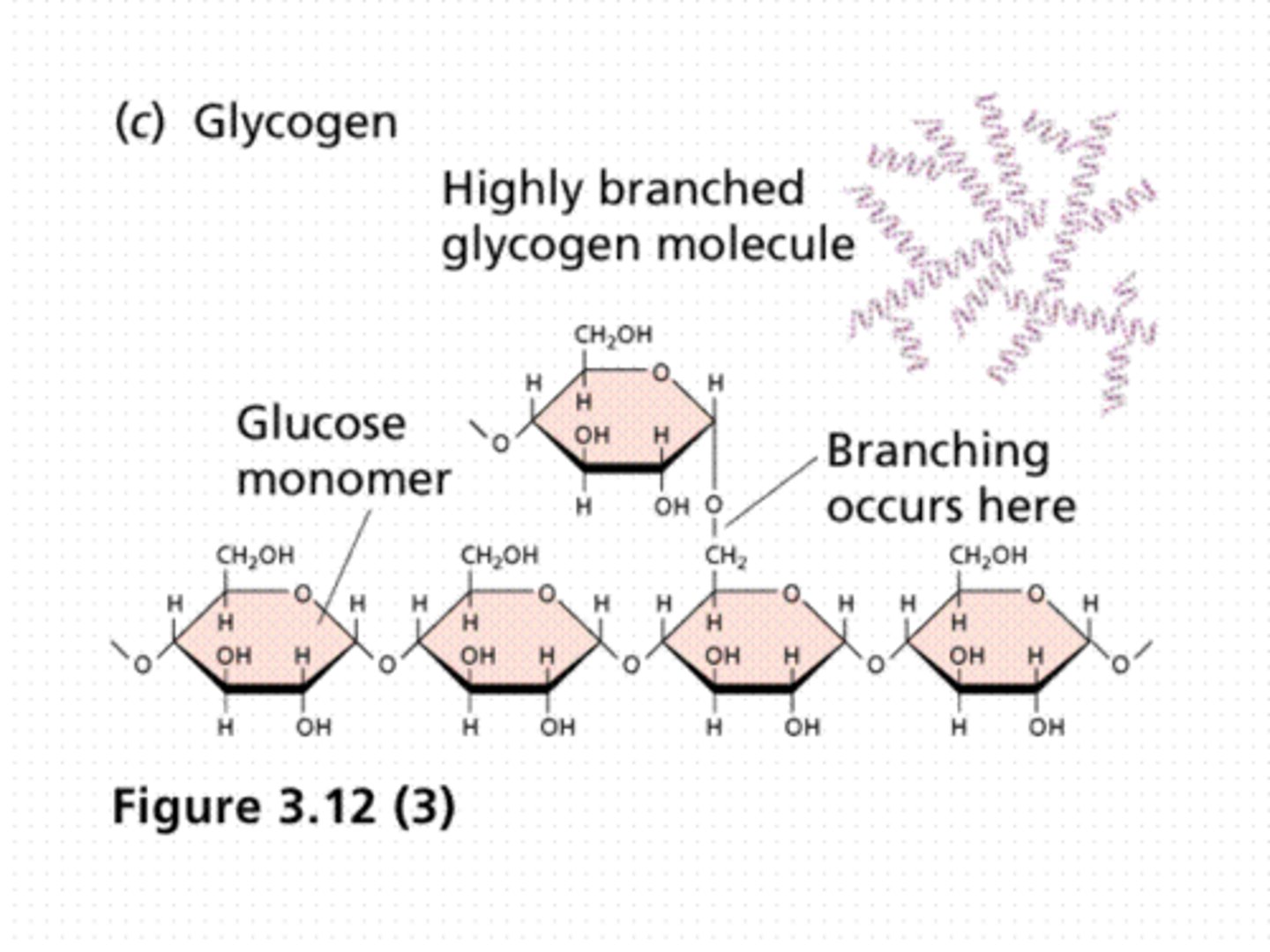
What is the structure of cellulose and how does it relate to its function?
A polysaccharide made up of chains of beta glucose molecules. They form a straight, unbranched molecule. The chains run parallel to each-other, crosslinked by H bonds. The beta glucose molecules flip in a way that starts another chain underneath to form straight parallel chains, held together with hydrogen bonds but between each molecule within the chain, there are glycosidic bonds.
- Main component of a cell wall so provides rigidity making it a valuable structural material.
- It is important in maintaining leaves and cells in a turgid state so provides the maximum surface area for photosynthesis.
- It prevents cell bursting when water enters through osmosis.
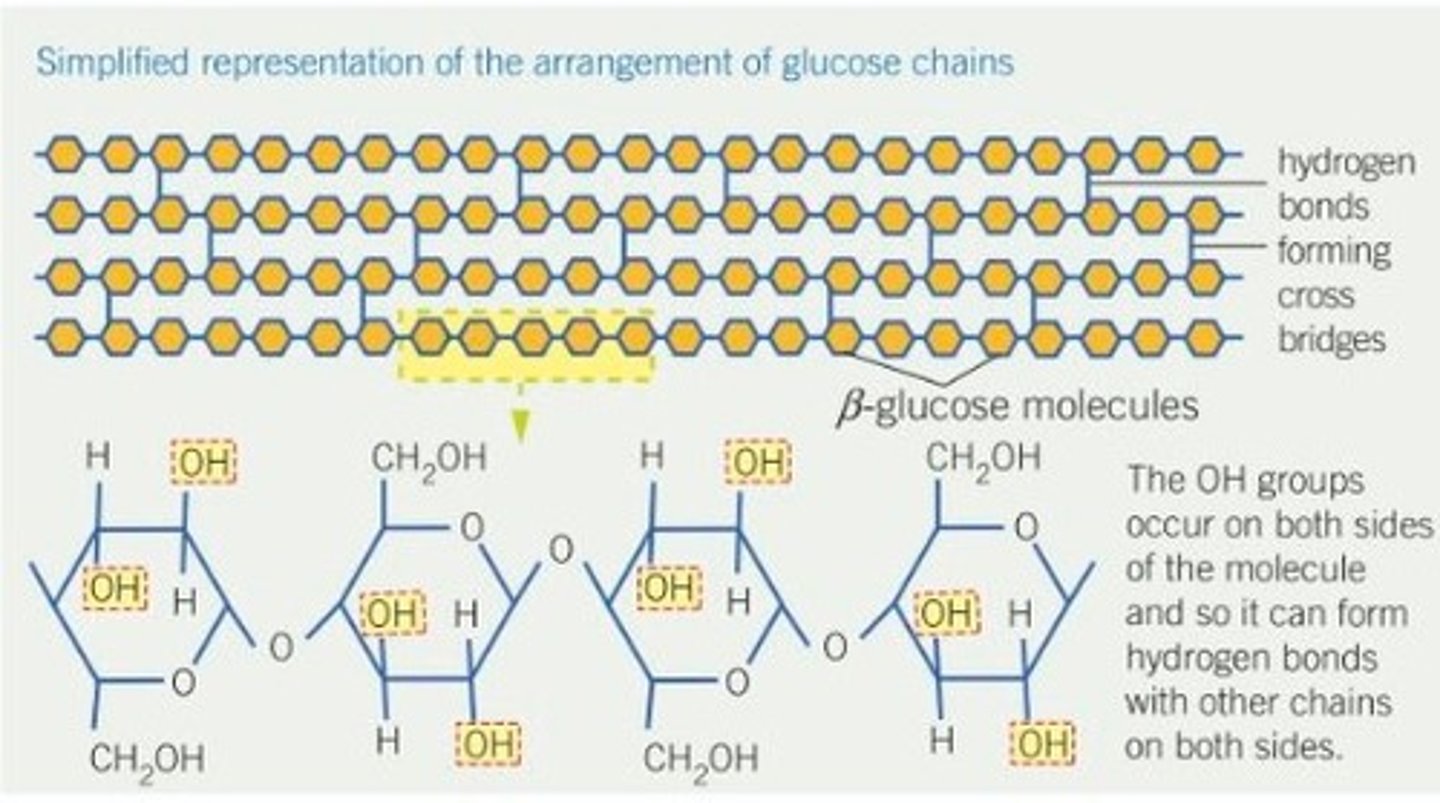
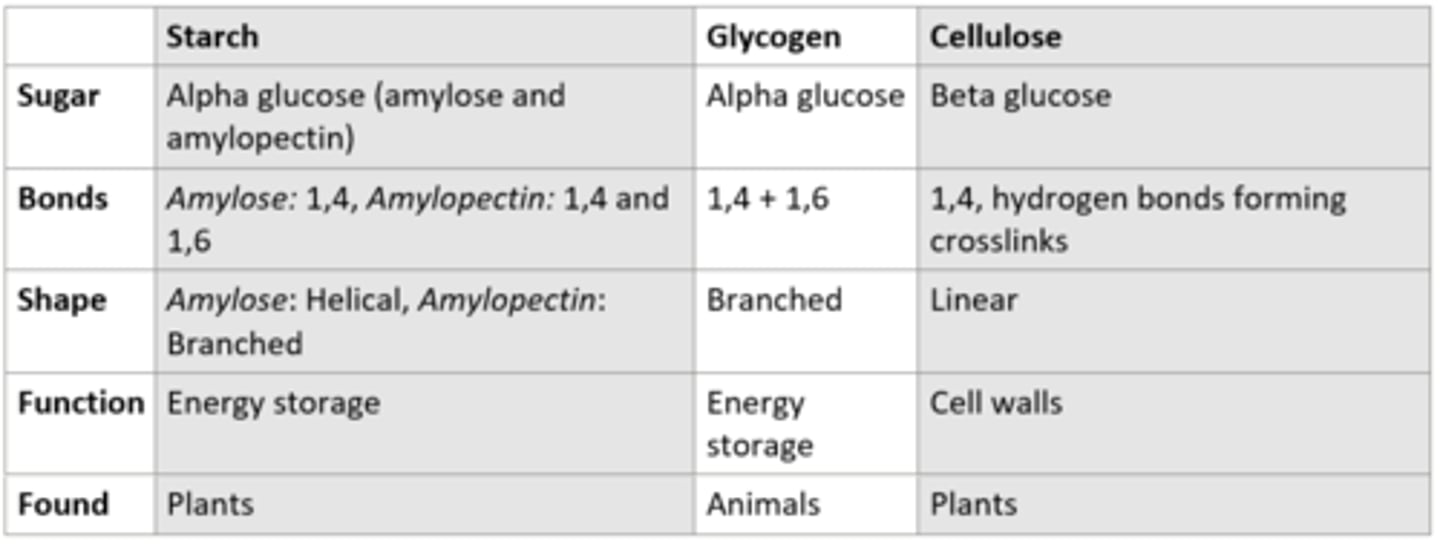
Table describing the properties of glycogen, starch, and cellulose:
What are glycoprotein composed of?
They contain a carbohydrate/sugar component and protein/amino acid component.
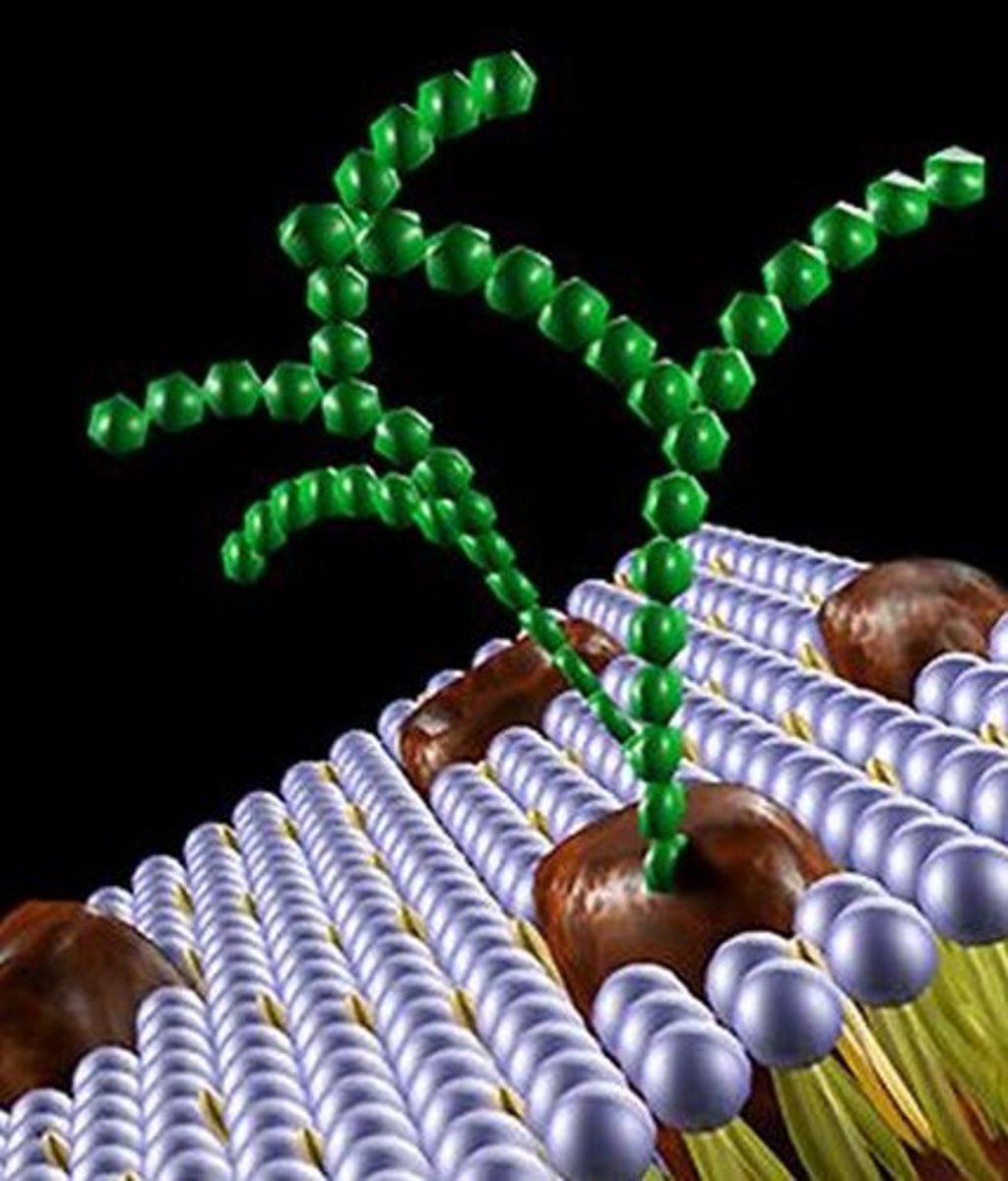
Where are glycoproteins found
They are found in the cell membrane. The protein component is embedded into the membrane with the carbohydrate chain coming off of the top.
What is the function of glycoproteins?
They are marker molecules (antigens) that are important in cell recognition (self vs non-self) and signalling.
What is an example of the role of cell-cell recognition in glycoproteins?
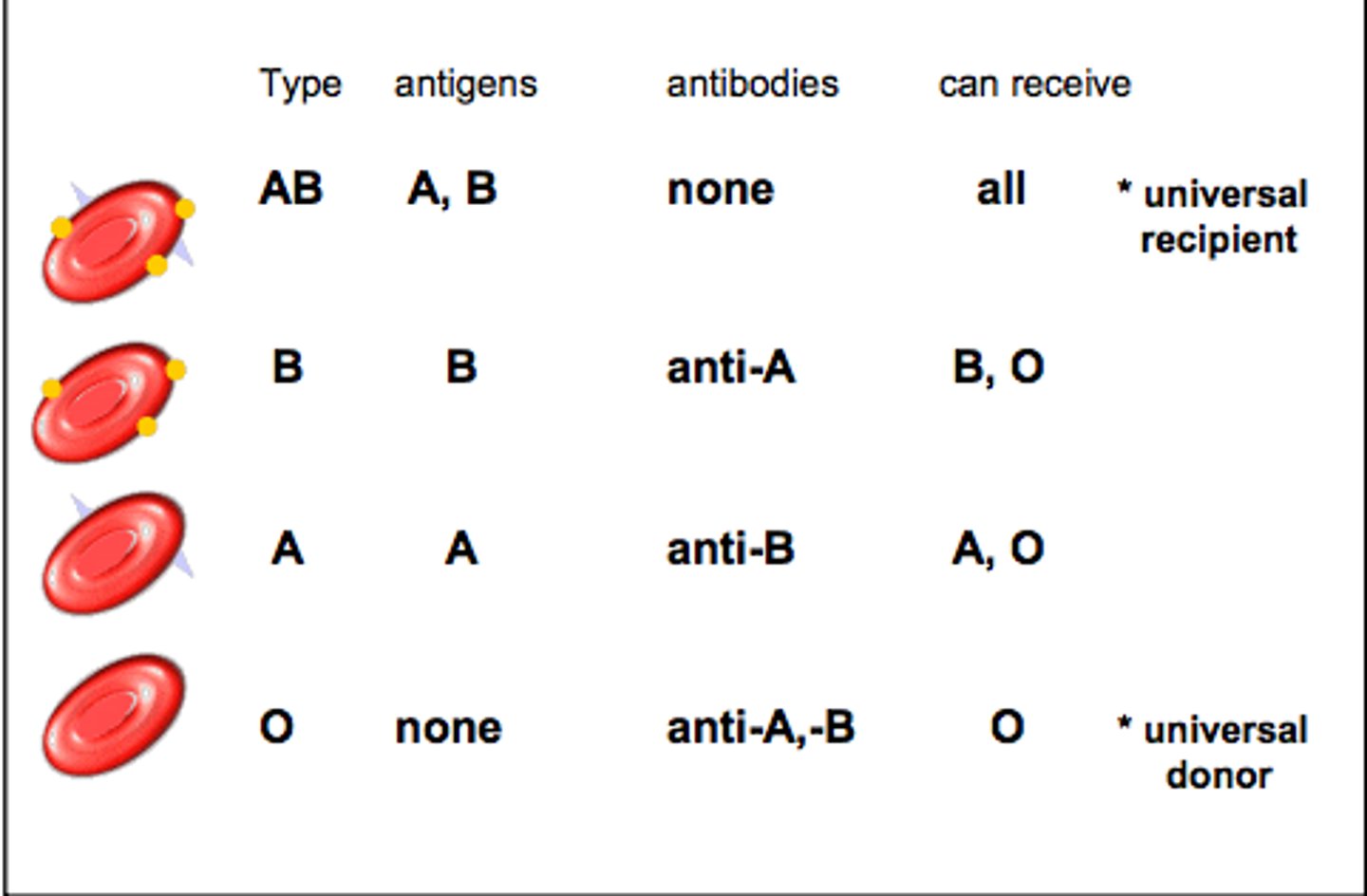
Red blood cells: they have glycoprotein antigens on their cell membrane surface.
- Type O have no A or B antigens.
- Type A have only A antigens.
- Type B have only B antigens.
- Type AB have both A and B antigens.
If blood cells with unfamilair antigens enter the bloodstream, they will not be recognised, be identified as 'non-self' cells, and attacked.
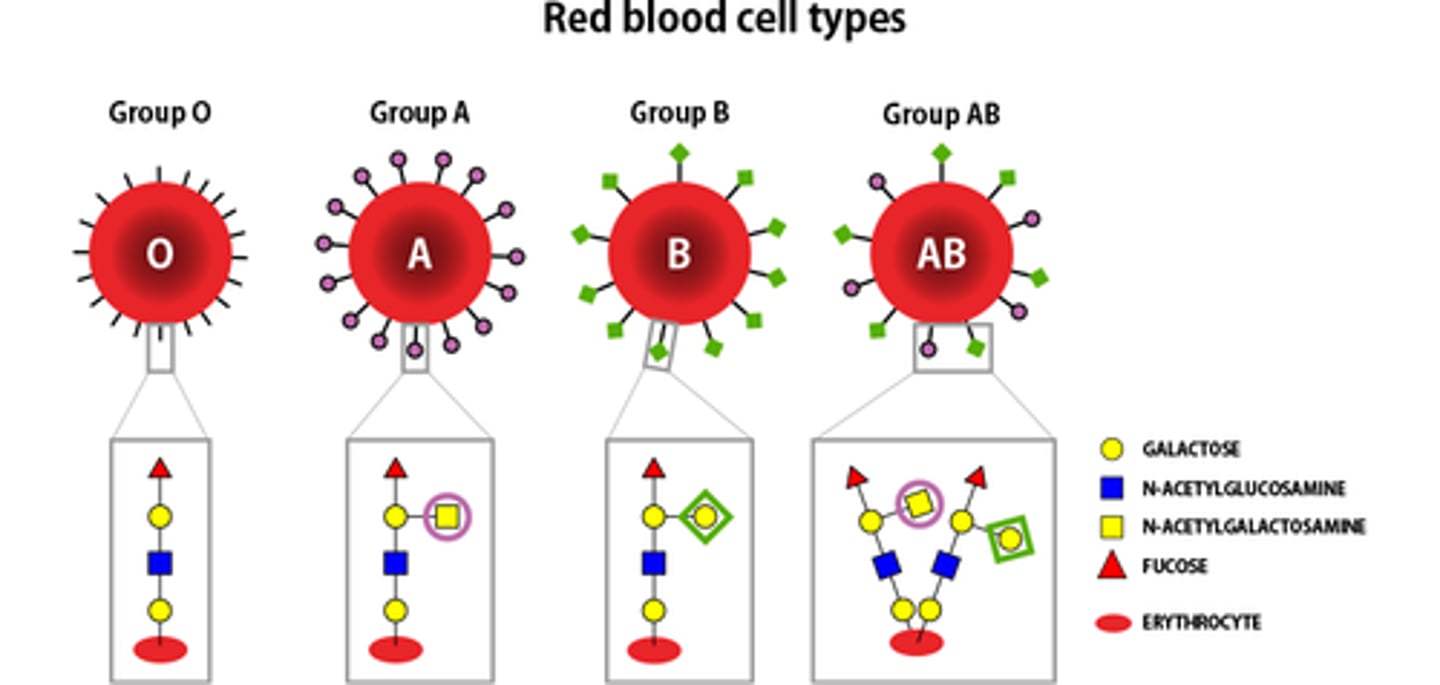
Which blood type can donate/receive from which other type?
- Blood type O is the universal donor.
- Blood type AB is the universal recipient.
Lipids
What are the 4 types of lipids?
- Fats
- Oils
- Waxes
- Steroids
What elements are lipids made up of?
Carbon, hydrogen, oxygen. They have a lower proportion of oxygen compared to carbohydrates.
What two components are lipids composed of?
- Glycerol
- Fatty Acids
What are the properties of lipids?
- Non-polar, hydrophobic molecules so do not dissolve well in water.
- Dissolve in non-polar solvents e.g., ethanol.
- Oxidised in respiration to drive the production of ATP.
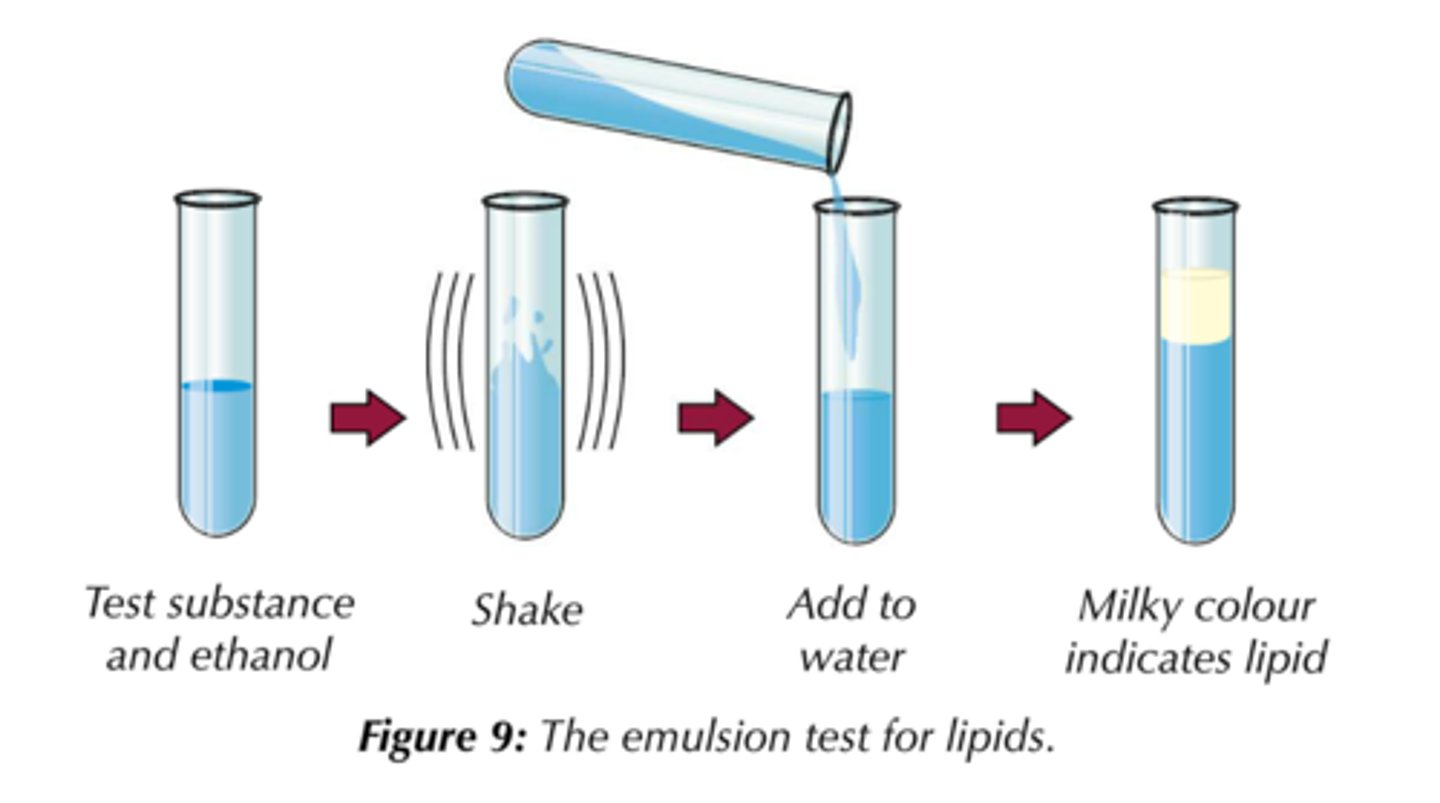
What is the test for lipids?
1. Add the lipid to ethanol (and shake)
2. Add water to the solution. If a lipid is present, the solution will turn cloudy white.
What is the chemical formula of glycerol?
C3H8O3
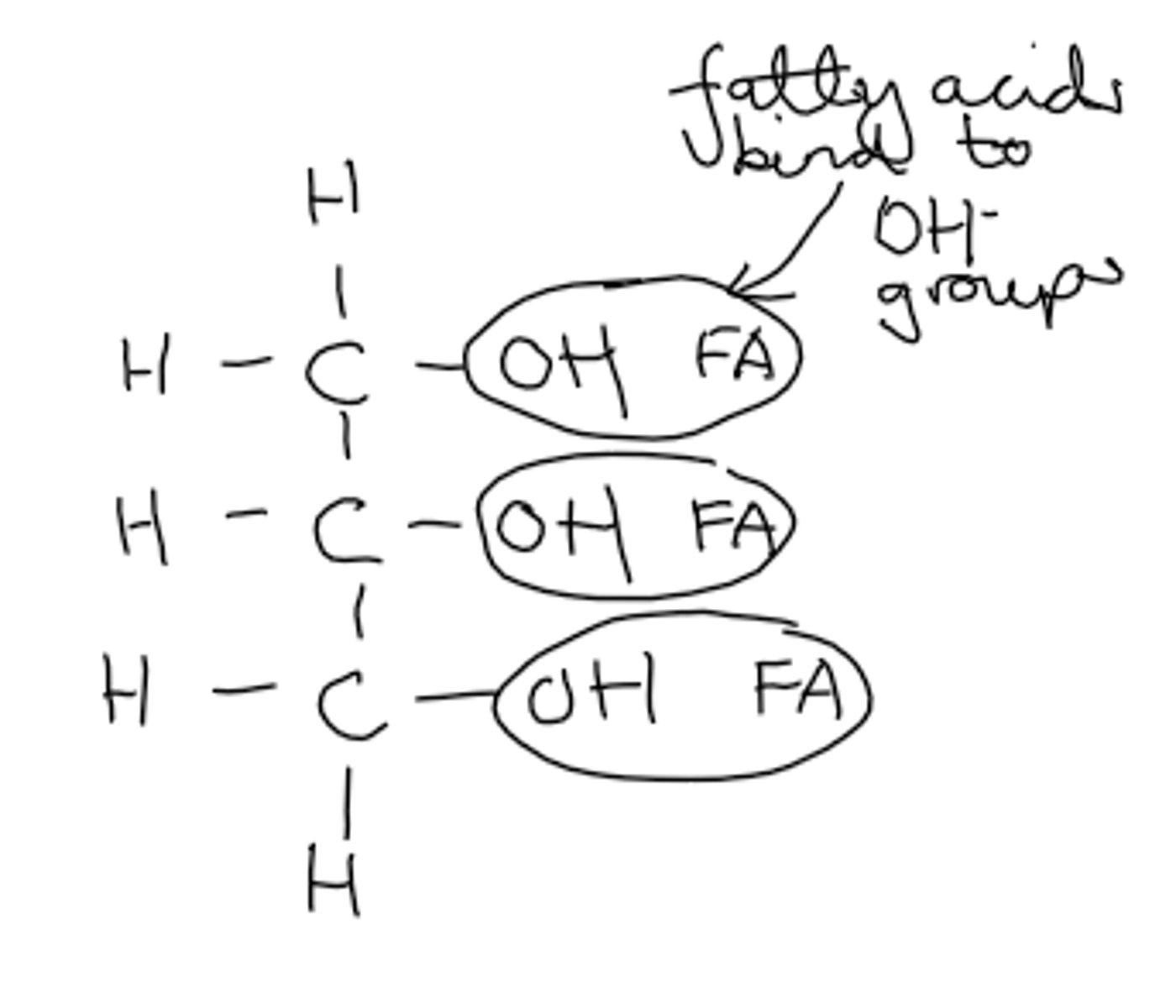
What is the structure of glycerol?
- 3 carbons with 3 hydroxyl groups and 5 hydrogens
- Always saturated bonds
- Its hydroxyl (OH) functional group is involved in its bonding
What is the structure of fatty acids?
- Long chains (of different lengths) of hydrocarbons with a carboxyl acid group (COOH)
- Their carboxyl acid group is involved in bonding
- The variable section of hydrocarbons is called the R group
- They can be saturated (with hydrogen) or unsaturated.
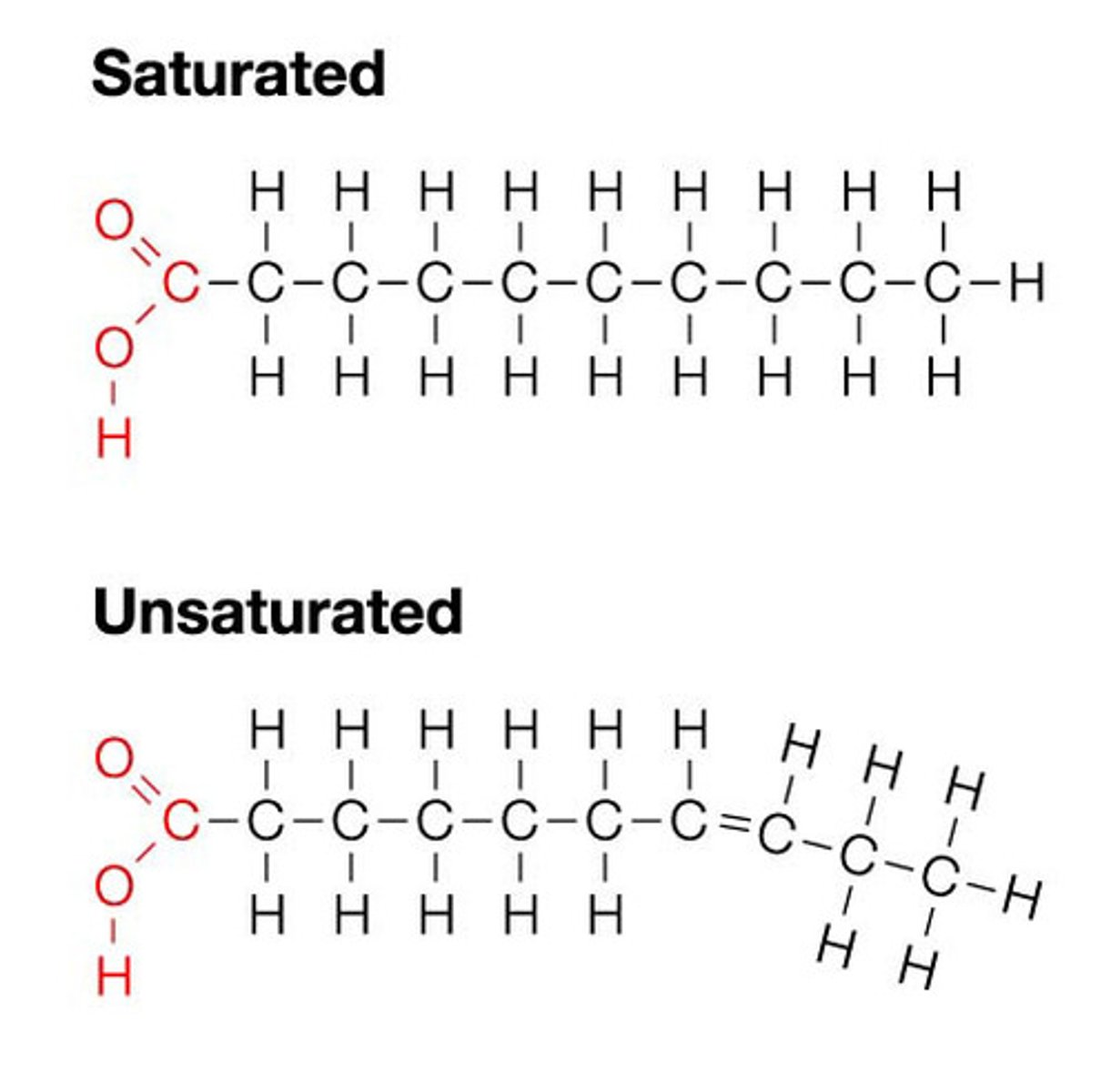
What are the 3 different types of fatty acids, their properties, and an example of a triglyceride containing them?
- Saturated (no double bonds): high melting point, solid at room temperature, straight, strong carbon chain, animals use them to store excess energy.
E.g. fats, lard, butter
- Monounsaturated (1 double bond): lower melting point, liquid at room temperature, carbon chain with 1 kink, some animals store energy in this form.
E.g., oil
- Polyunsaturated (more than 1 double bond): low melting point, liquid at room temperature, carbon chain with multiple kinks.
E.g. oil
What is the structure of a triglyceride?
Glycerol + 3 fatty acids
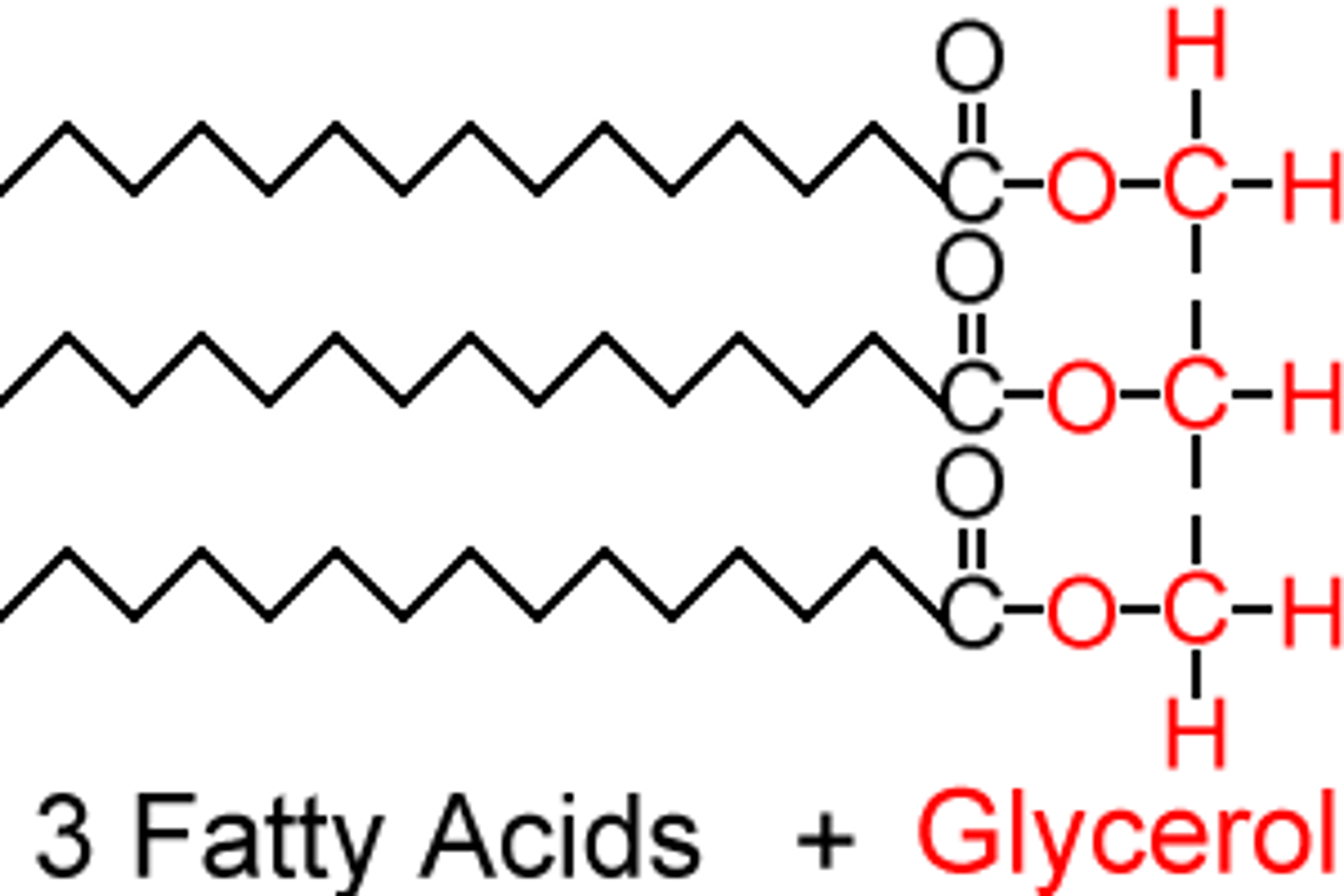
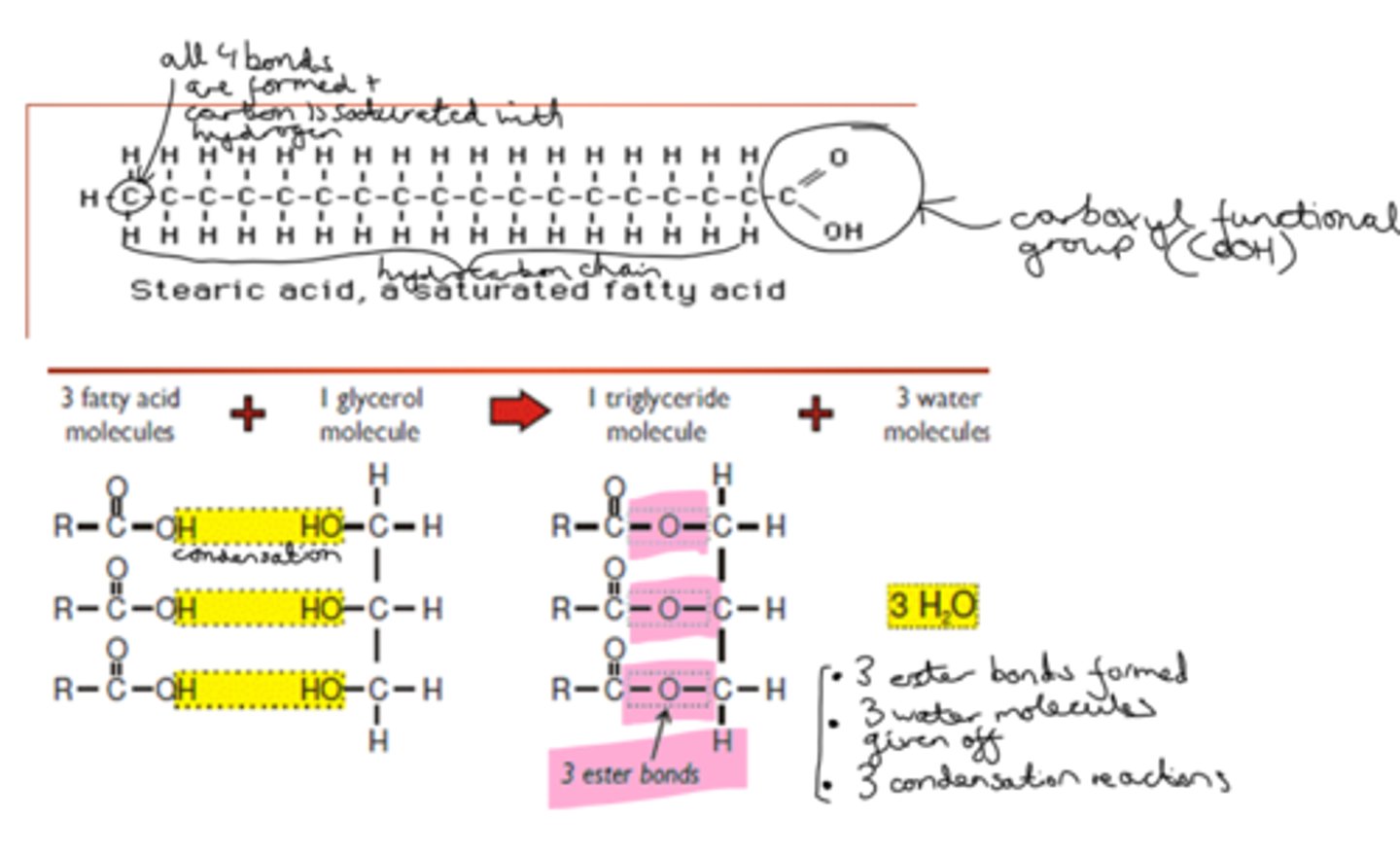
How is a triglyceride formed?
When a triglyceride is formed, there is a condensation reaction between the carboxyl group of each fatty acid and the hydroxyl functional groups of the glycerol molecule, evolving 3 H2O (water) molecules and forming ester (covalent C-O) bonds between the glycerol and fatty acid molecules.
What is the structure of a phospholipid?
Glycerol, 2 fatty acids, and an inorganic phosphate group
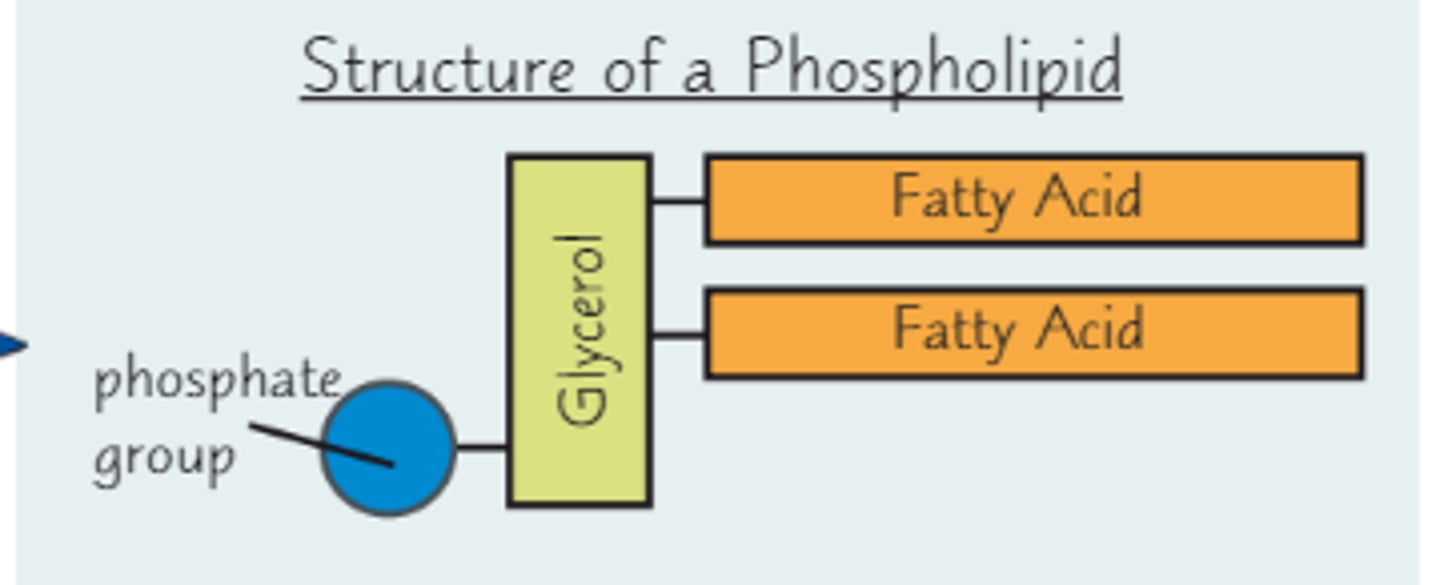
How is a phospholipid formed?
When a phospholipid is formed, there is a condensation reaction between the 2 fatty acids and the phosphate group and the hydroxyl functional groups of the glycerol molecule, evolving 3 H2O (water) molecules and forming ester (covalent C-O) bonds between the glycerol and fatty acid/phosphate molecules.
How much energy do lipids store relative to the same mass of carbohydrate?
They store 3x as much energy
Where are lipids stored?
In adipose tissue
What is adipose tissue?
Adipose tissue is composed of cells that store fat in the form of triglycerides. The quantity of triglycerides that is stored is determined by the organism's caloric intake compared to the calories burned.
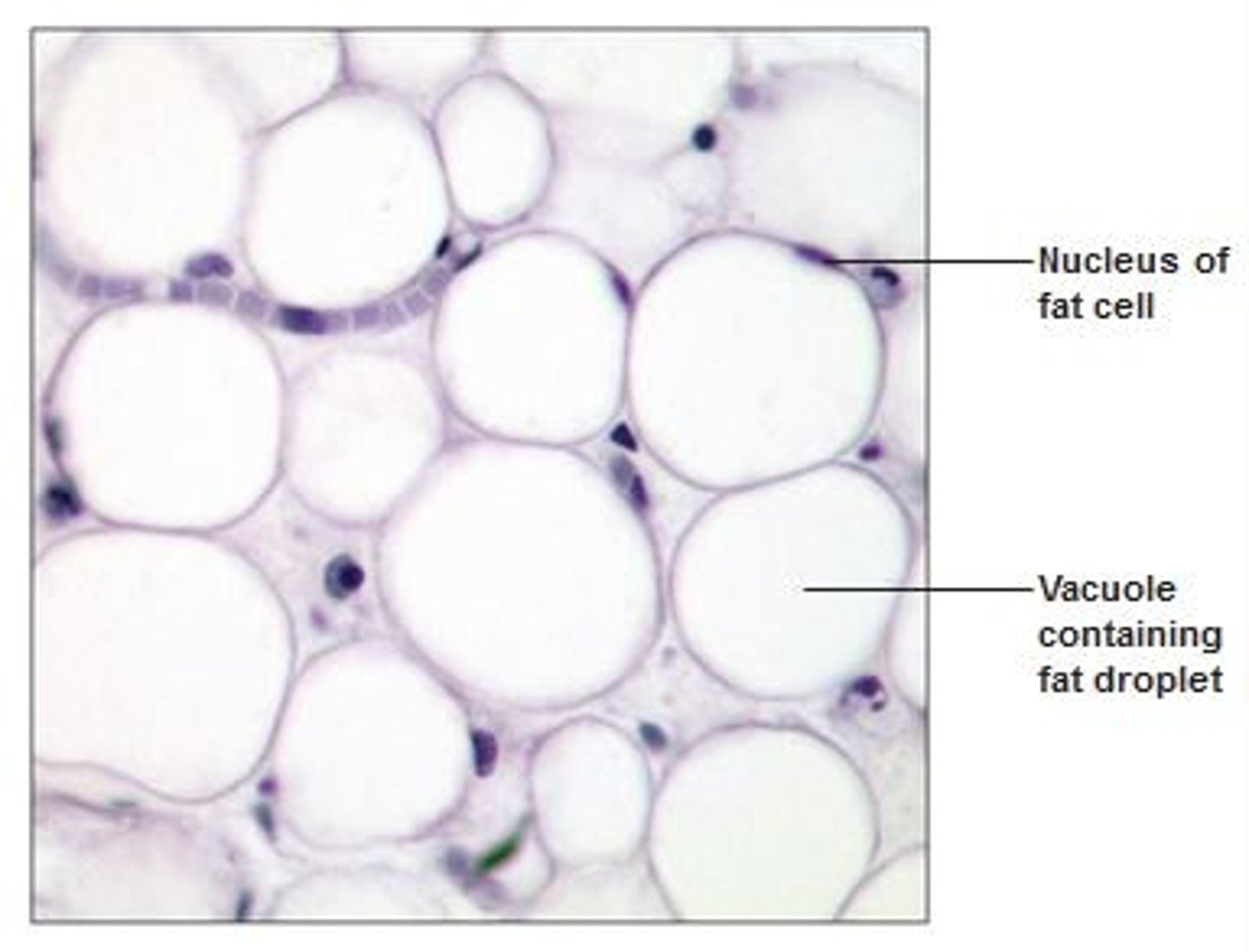
Where is adipose tissue found?
In birds and mammals it is found between the skin and muscles.
How do triglycerides supply energy?
They supply energy when sufficient foods are not available.
They undergo hydrolysis reactions and the products (glycerol and fatty acids) are made available for energy in the process of cell respiration.
Why are they most useful for long-term energy storage?
They are useful for long-term energy storage because they are insoluble in body fluids and thus will not move from their adipose storage sites.
How are lipids useful to organisms?
- Waterproofing ( oils in fur/feathers) which means they don't become water-logged and water simply runs off when the animal leaves the water. The same applies for waxes on plant leaves.
- Good insulators, retaining heat in the body. This is particularly advantageous for animals that live in an extreme environment.
- Low in density, allowing aquatic animals to float and be buoyant.
Why were lipids useful in the past to humans?
Lipids were useful in the past as there wasn't a plentiful environment and lipids ensured that humans could store energy long-term for survival.
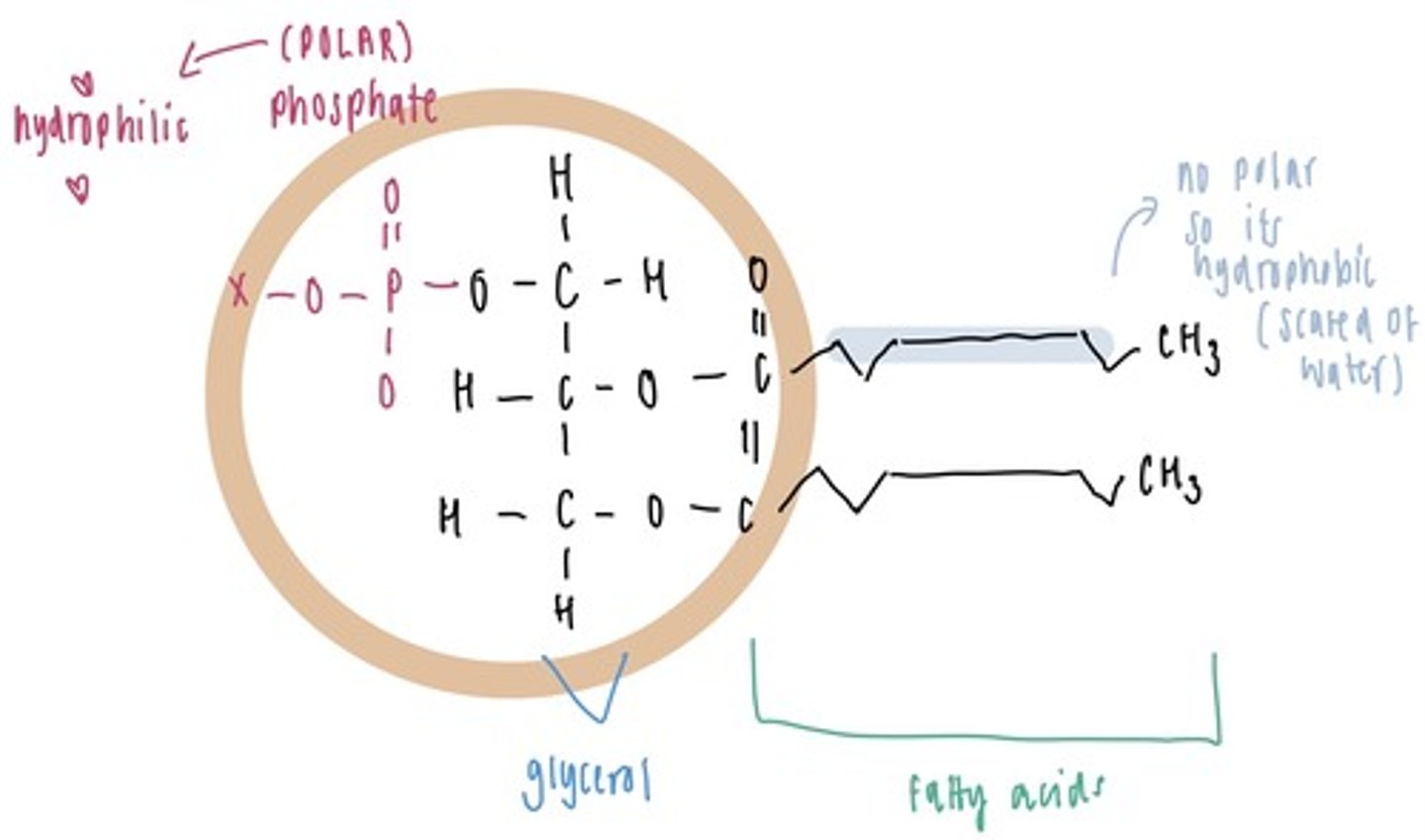
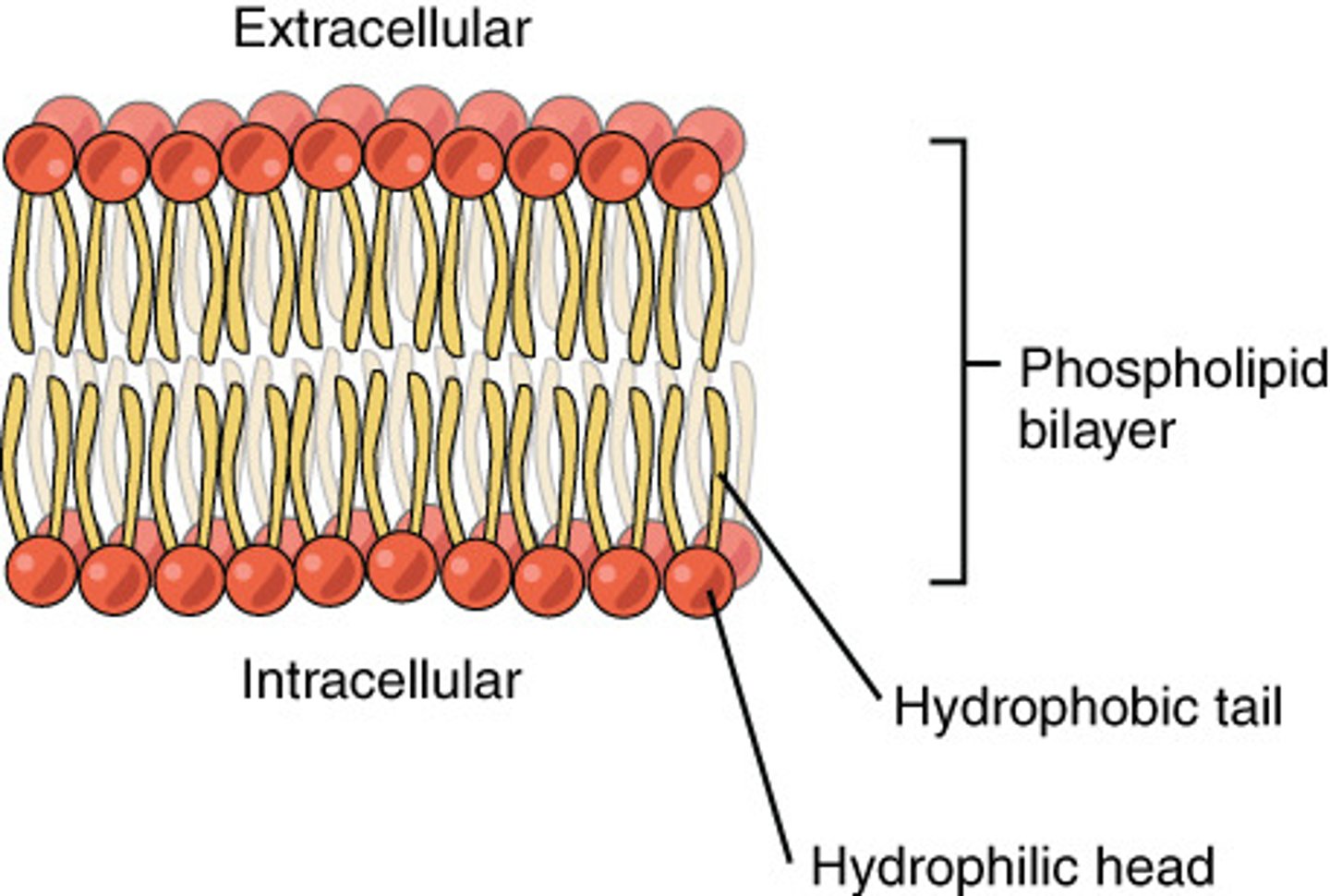
What type of molecule is a phospholipid?
Amphipathic as it has both hydrophilic and hydrophobic regions. The phosphate group is polar and the hydrocarbon tail is non-polar.
How do the properties of phospholipids allow for the formation of the cell membrane?
In an aqueous solution, phospholipids arrange themselves in a bilayer where the hydrophobic fatty acid tails extend towards each other in order to avoid the aqueous solutions inside and outside of the cell. The polar phosphate groups are attracted to the aqueous solutions and so arrange themselves on the outside of the bilayer.
What are hormones?
Chemical messenger molecules produced by a variety of glands in the body. They are released into the bloodstream and have access to all body tissues.
What are steroid hormones?
Steroid hormones are made from cholesterol. Cholesterol is primarily a hydrocarbon molecule.
What is the structure of steroid hormones and 2 examples?
All steroids have 4 connected rings structures and are formed from cholesterol.
2 examples are testosterone and oestradiol (oestrogen)
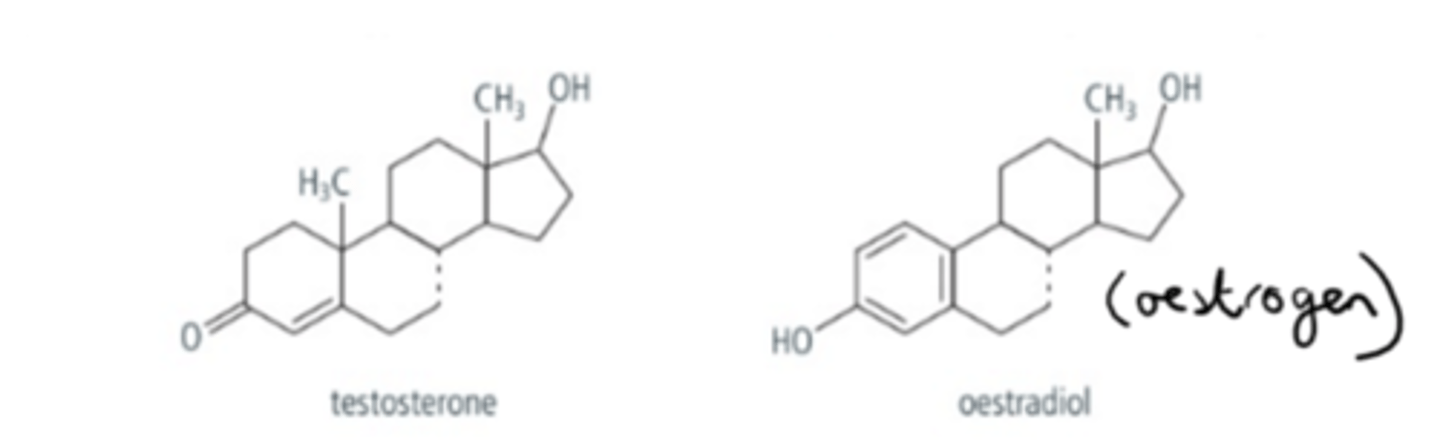
What is similar about oestradiol and testosterone?
- Both are produced by gonadal tissue
- Both are involved in the development of primary and secondary sex characteristics beginning at puberty
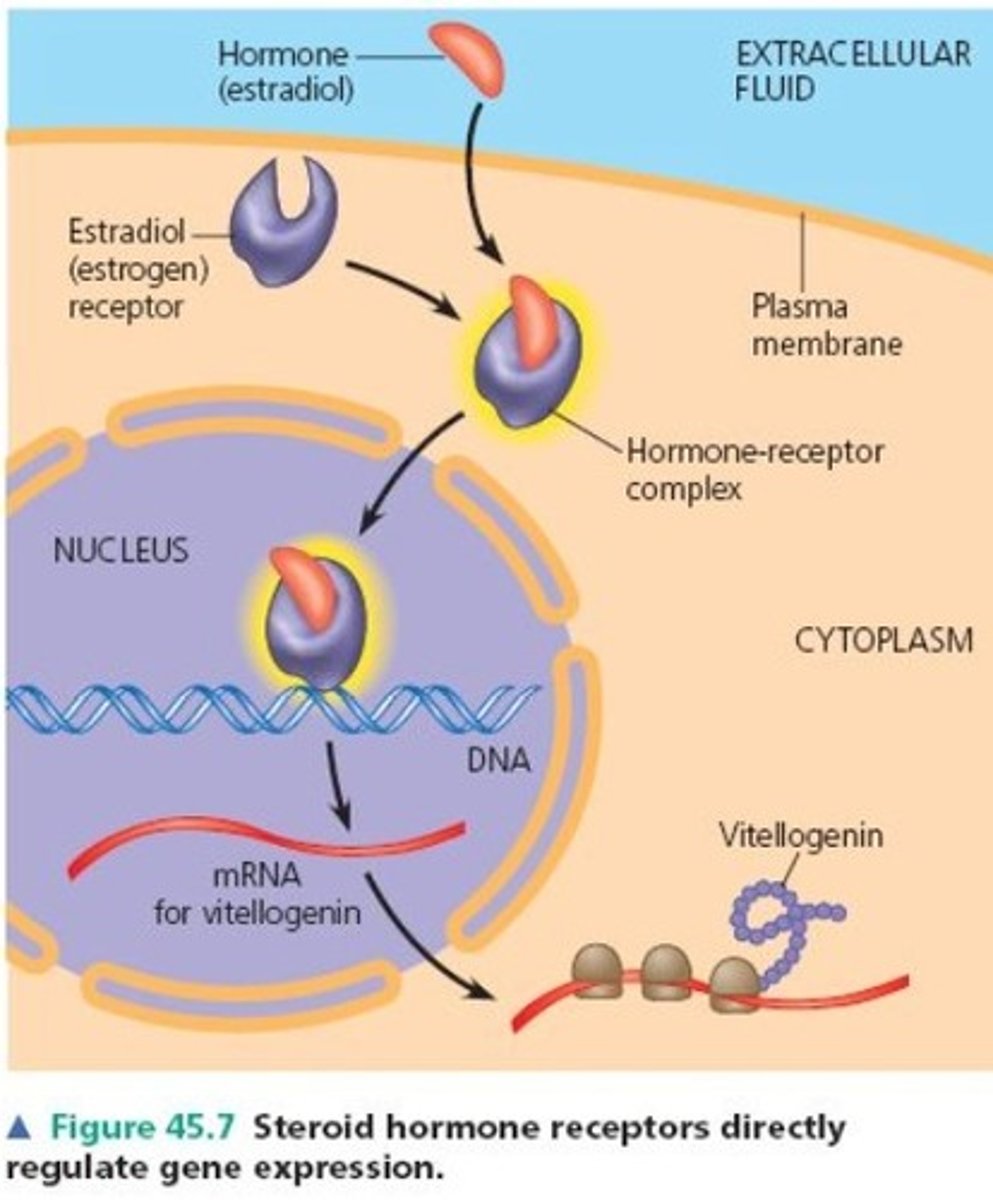
How do steroid hormones work?
1. They enter their target cells through the phospholipid bilayer (since they are lipids so soluble in other lipids) then the nucleus through the nuclear membrane.
2. Once inside, they direct the process of transcription, leading to the production of mRNA molecules.
3. The mRNA molecules travel to the ribosomes via the cytoplasm for translation.