BIOCH 200 - Introductory Biochemistry
1/44
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
45 Terms
Iron (Fe)
Important trace element.
Binds oxygen.
Moves electrons (especially through the electron transport chain).
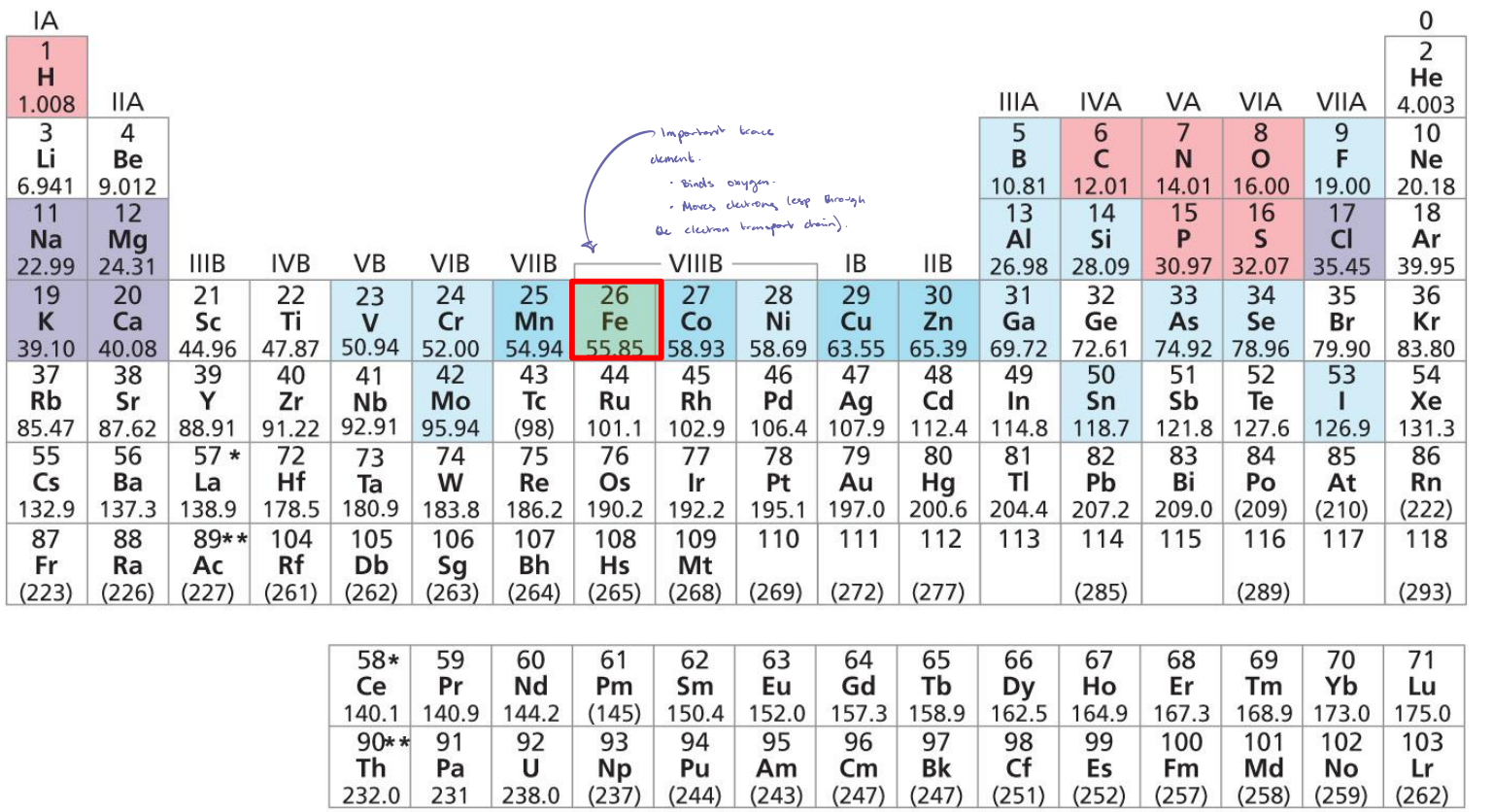
Biomolecules!
H2O, CO2, NH3, O2, N2 → turn into amino acids (monomer), carbohydrates (monomers), nucleotides (monomers), lipids (not monomers.
In its poly- form, amino acids are proteins, carbohydrates are polysaccharides, and nucleotides are nucleic acids.
Polysaccharides → ribosomes, chromatin, membranes, etc.
Biomolecules Flowchart
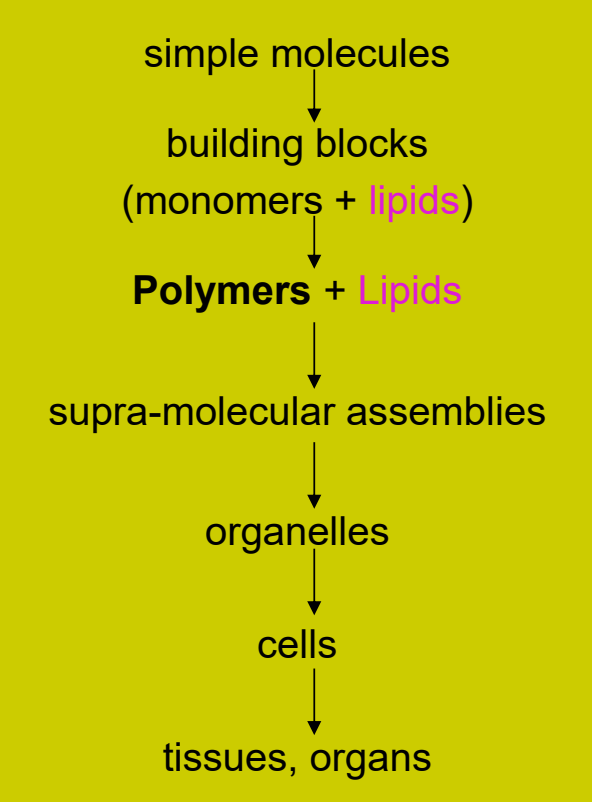
Monomers
Is a molecule that can be covalently bonded to other identical/similar molecules to form a polymer.
i.e. Amino acids, nucleotides, monosaccharides.
The start and end always in a polymer → b/c of covalent bonds.
Amino Acids
Soluble in water.
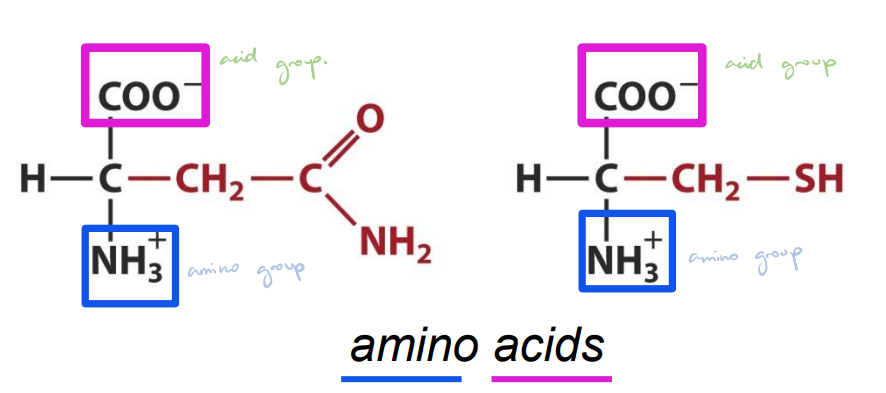
Carbohydrates
Soluble in water.
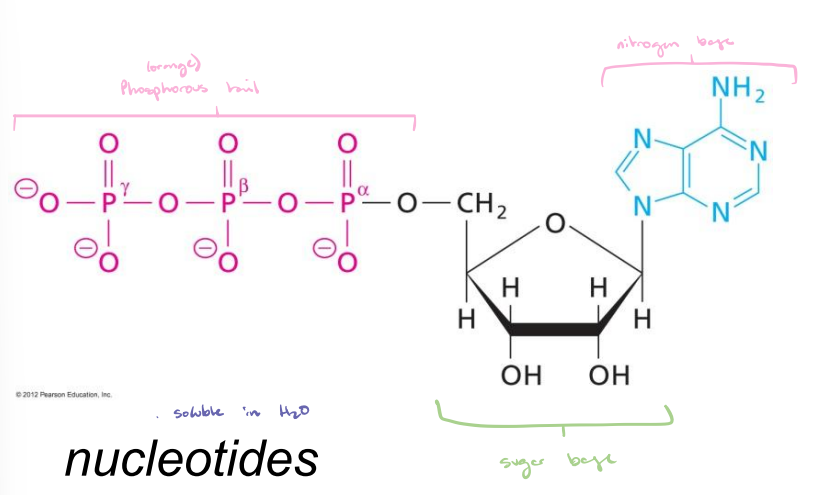
Nucleotides
Soluble in H2O.
Phosphorous tail and nitrogen base attached to a sugar base.
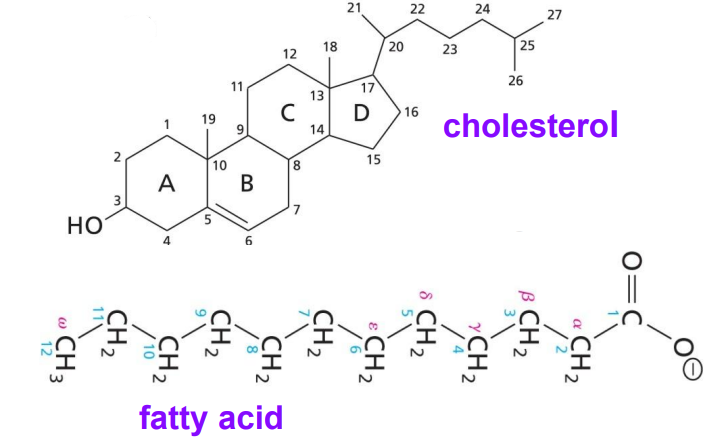
Lipids
Is not soluble in water.
Carbohydrates — Key Features
General formula: (C • H₂O)ₙ; unbranched carbon chain where n ranges from 3–9.
Structure in solution: Typically exists in a ring structure.
Functional groups: n − 1 hydroxyl groups and always 1 carbonyl group.
Carbonyl details: Carbonyl group can be an aldehyde or ketone, usually on carbon 1 or 2; polyhydroxy aldehydes and ketones.
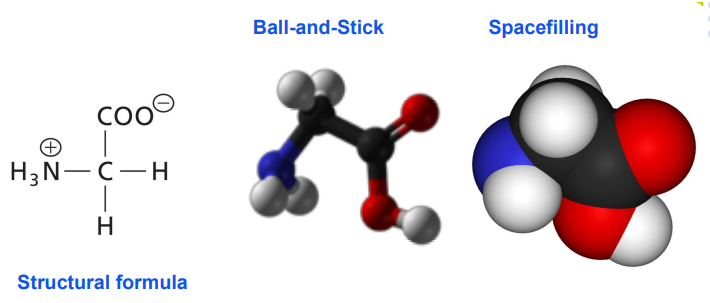
Biomolecules
Oxygen – red.
Carbon – grey/black.
Hydrogen – white.
Nitrogen - blue.
S – yellow.
P – orange.
Organic Compounds
A carbonyl group is simply C=O.
Memorize!
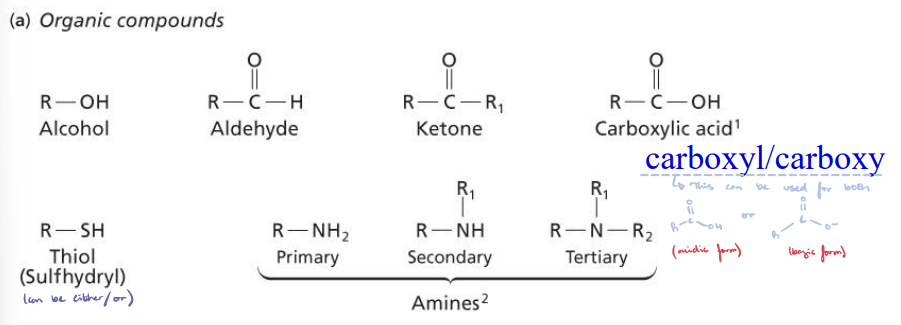
Functional Groups
Nitrogen/oxygen groups: Amino group (amine) and hydroxyl group (alcohol).
Sulfur and carbonyl groups: Sulfhydryl group (thiol) and carbonyl group (aldehydes and ketones).
Carboxyl groups: Carboxyl/carboxy; carboxylic acid (H) and carboxylate (conjugate base).
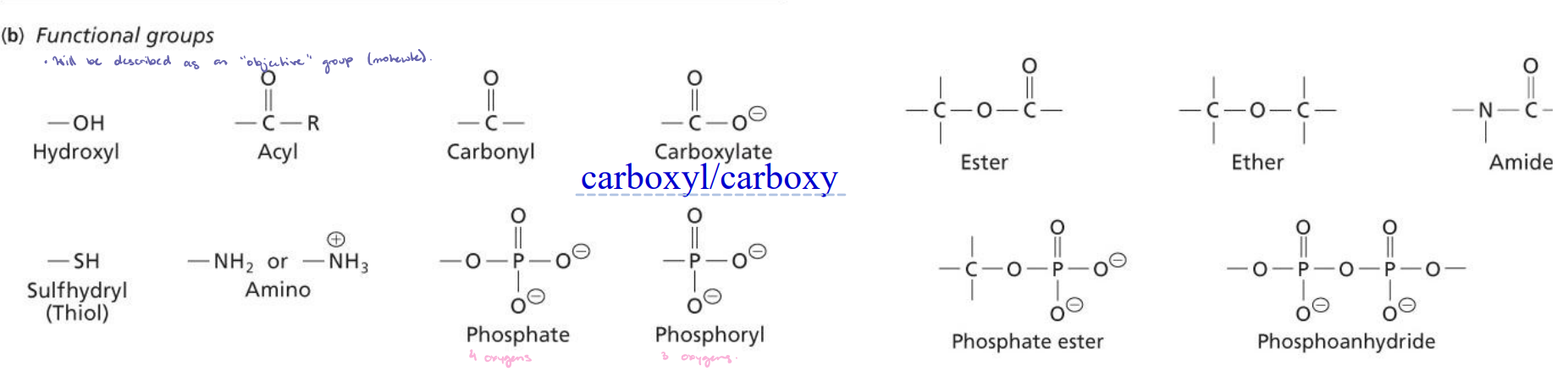
Linkages in Biochemical Compounds
Will be described as a linkage when reactions occur.
Under most biological conditions, carboxylic acids exist as carboxylate anions.
When you see “POP” it is a phosphoanhydride.
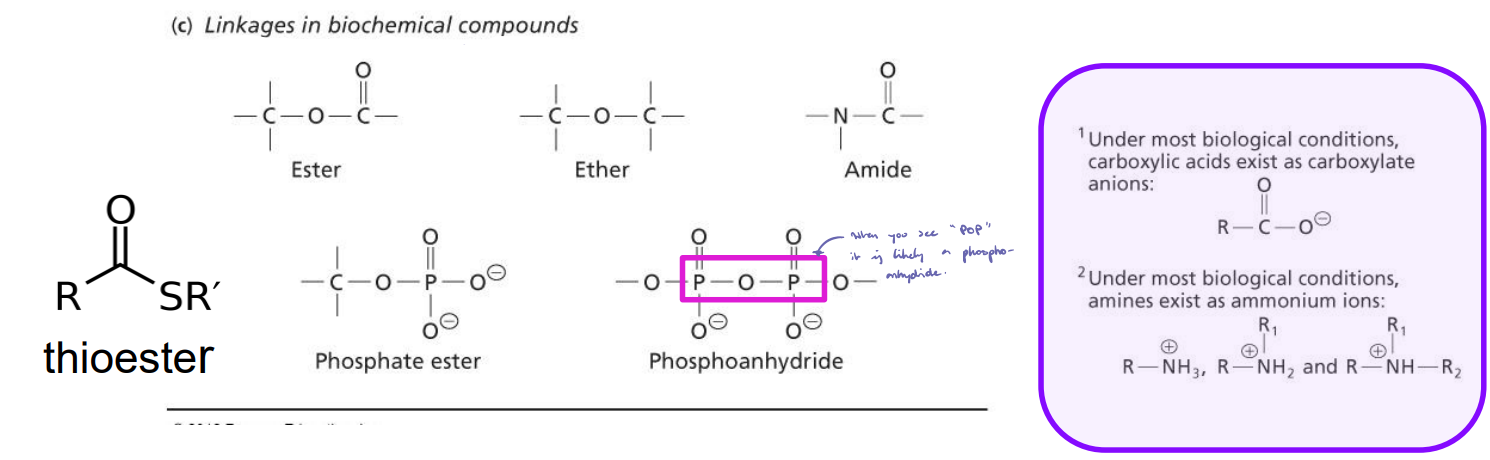
Acyl Group
Formation: Formed by removing one or more hydroxyl groups from an oxoacid (typically a carboxylic acid) during attachment to another molecule.
Structure: Carbonyl group (C=O) bonded to an alkyl group.
Function: Only formed when two molecules are linked together.

Linkages: Esters
Linkage between carboxyl group and a hydroxyl group.
A condensation reaction and a dehydration reaction.
H2O is formed as a byproduct.
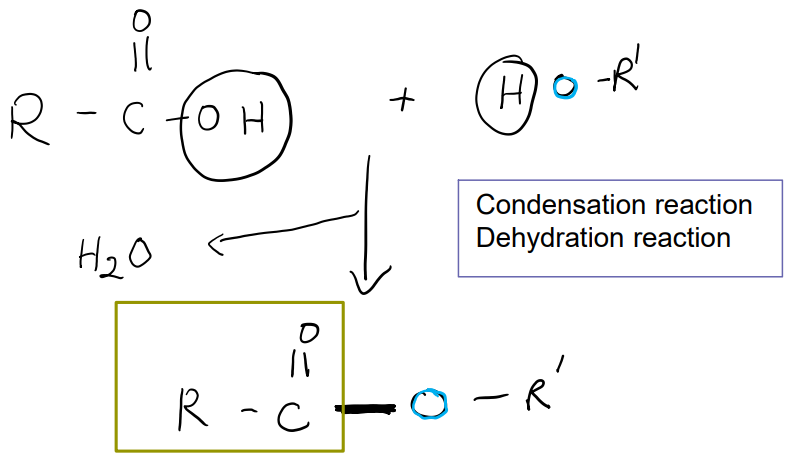
Linkages: Amides
Linkage between a carboxyl group and a amino group.
Dehydration reaction and condensation reaction.
Polymer
Monomer that is part of a polymer.
They have “directionality” - all covalent bonds are in the same orientation.
Residue
Monomer which is a part of a polymer.
Is not a monomer itself.
Monomers and Polymers
Amino acids → protein.
Has a peptide bond.
Nucleotides → nucleic acids.
Has a phosphodiester bond.
Electronegativity & Dipoles
Electronegativity: Some atoms pull electrons stronger than others.
Least → most electronegative: H, C, S, N, O.
Bond effect: Covalent bonds between atoms with different electronegativities create a permanent dipole.
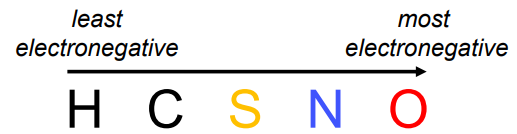
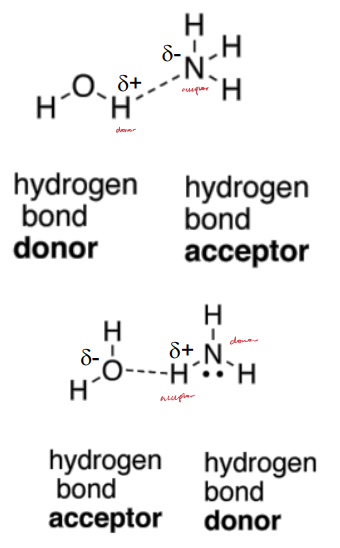
Hydrogen Donor
A hydrogen bonded to an electronegative atom (i.e. O, N, or S).
Hydrogen Acceptor
A lone pair of electrons associated with an electronegative atom.
Must have a lone pair (O, N).
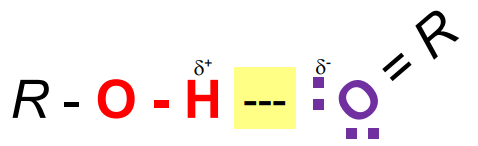
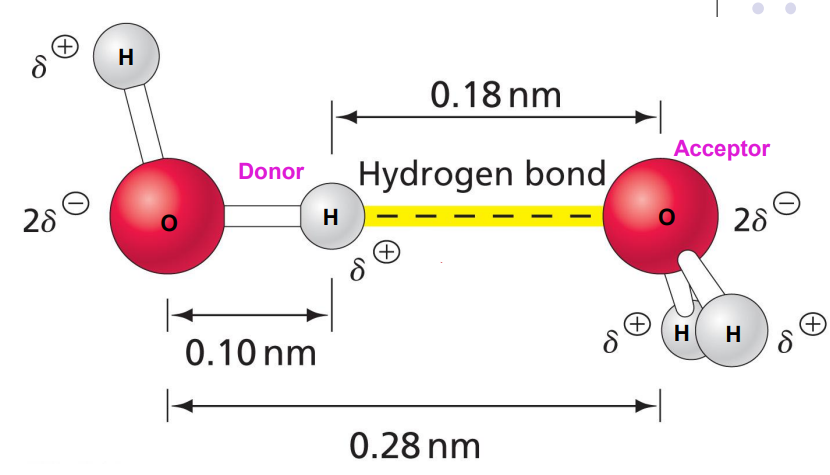
Hydrogen Bond
H-bond interaction is almost double the length of the covalent bond ∴ weaker bond.
Each H2O molecule can form 4 H-bonds, two as donors and 2 as acceptors.
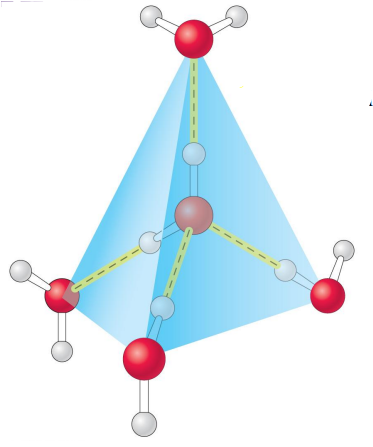
Ice
In ice each water molecule forms 4 H-bonds.
Hydrogen bonds in ice are most stable than hydrogen bonds in water.
Acid–Base Behavior of Water
Bonding: ~3 H-bonds per water molecule on average.
Roles: Forms 2 H-bonds as an acceptor and 2 as a donor.
Acid–base: Acts as a weak acid by sharing (not fully giving away) its H as a donor; acting as a weak base means the H is not fully given away.
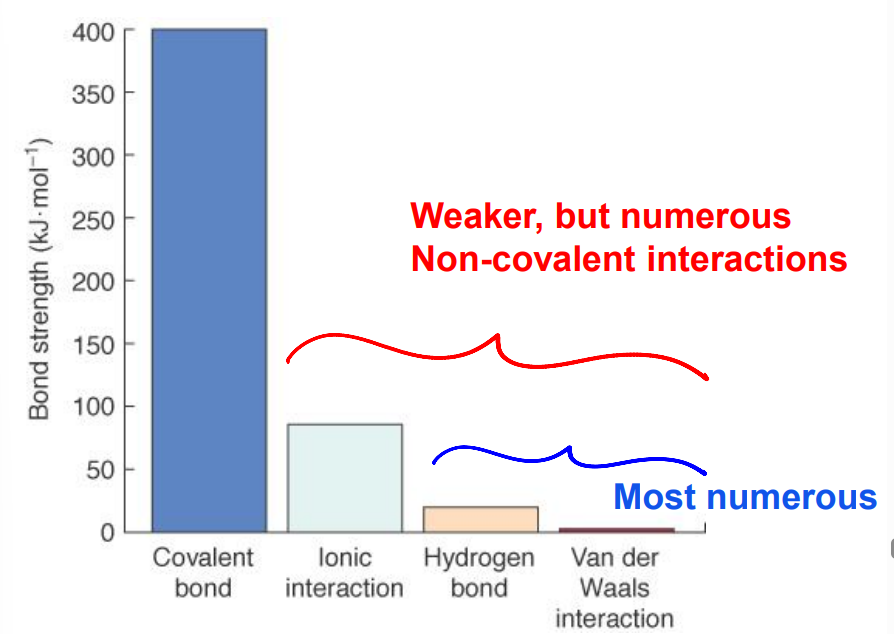
Non-Covalent Interactions
Electrostatic forces: Ionic interactions (− ↔ +); NEVER called an ionic bond; generally stronger.
Hydrogen bonds: Dipole–dipole interactions.
Van der Waals: Lack H in their bonds; include dipole–dipole interactions and LD forces.
Charge nature: Hydrogen bonds and van der Waals interactions are non-covalent associations between neutral molecules; no full ± charge, but form δ⁺/δ⁻.
Dipole-Dipole Interactions
Between polar non-charged groups.
Weaker than H-bonds.
London Dispersion Forces
Between nonpolar molecules.
Weaker than dipole-dipole interactions.
Exhibited by all molecules.
Hydrophobic Effect
The tendency of water to minimize its contacts with nonpolar substances, thereby inducing the substances to aggregate.
Important in LD forces.
Will involve LDF.
Effect is always based on water’s whims.
These are hydrophobic interactions.
Bond Strength in Biological Molecules
An interaction may be weak.
However, if there is a high amount of LD forces, they become strong together.
H-Bond Donors/Acceptors
Donors: N-H, O-H, and S-H (S not as strong).
Acceptors: -O-, -N-, and -S- (S not as strong).
Hydrophobic Effect - Shell
Layer of constrained water molecules.
A shell of water forms around the non-polar molecules.
Unhappy H2O molecules.
Water loves to make/break H-bonds.
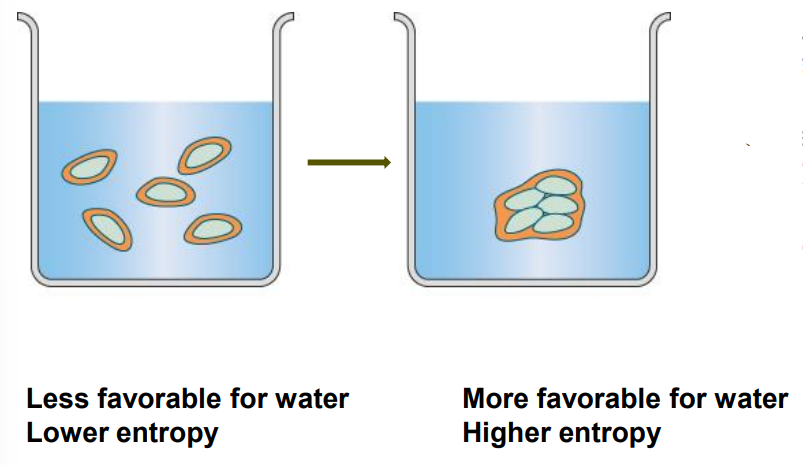
The Hydrophobic Effect - Entropy
Scattered non-polar molecules: Less favourable for water; lower entropy due to constrained water.
Grouped non-polar molecules: More favourable for water; higher entropy.
Mechanism: In water, non-polar compounds are forced to associate together.
Result: Less water is consumed; water shoves them together, increasing entropy.
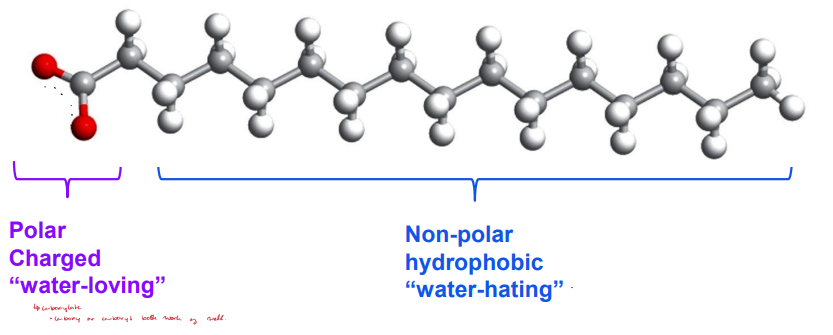
Amphipathic
Molecules experience hydrophilic interactions and the hydrophobic effect.
Used for bigger molecules (amphiphilic).
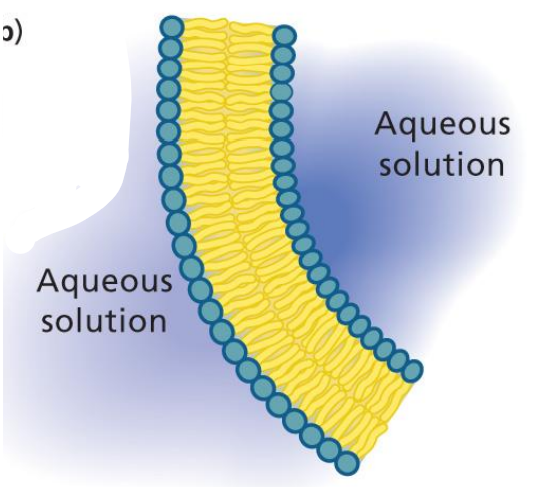
Micelle
Water shoves fatty acids into a spherical structure.
Aggregate to position polar parts close to water and nonpolar together away from water.
Self-assembling to a bilayer.
T2 Phage Structure
T2 phage is a virulent bacteriophage (virus) that infects Escherichia coli.
DNA is contained in the head. A protein coat forms the head, collar, and tail.
DNA is high in phosphorus (P), while the protein coat is relatively high in sulfur (S).
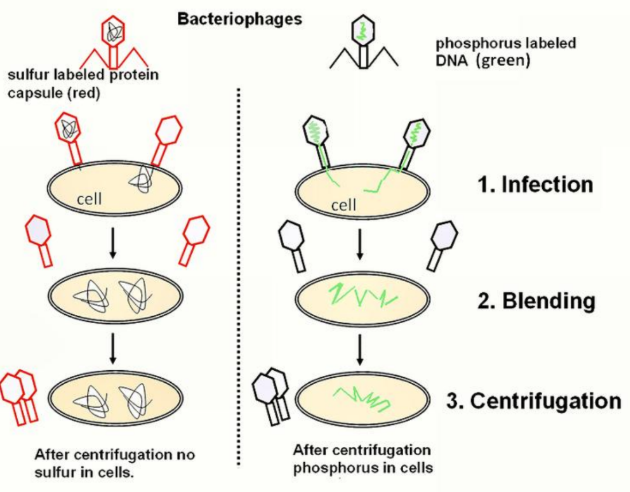
Hershey–Chase Experiment
Bacteriophages were labeled with sulfur to track protein or with phosphorus to track DNA, then allowed to infect cells.
After centrifugation, sulfur-labeled protein was not found in cells, while phosphorus-labeled DNA was found in cells.
Nucleotides and Their Polymers
Involved in nearly every facet of cellular life.
Storage and decoding of genetic information: DNA and RNA.
Act as enzymes: RNA.
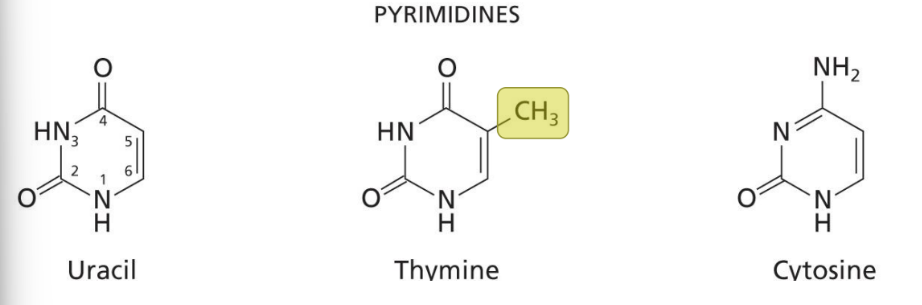
Pyrimidine
Smaller but longer name and must have two N’s in the ring.
All have a carbonyl at C2.
Uracil and thymine have carbonyls at C2 and C4; thymine has a methyl group at C5.
All bases are aromatic and heterocyclic.
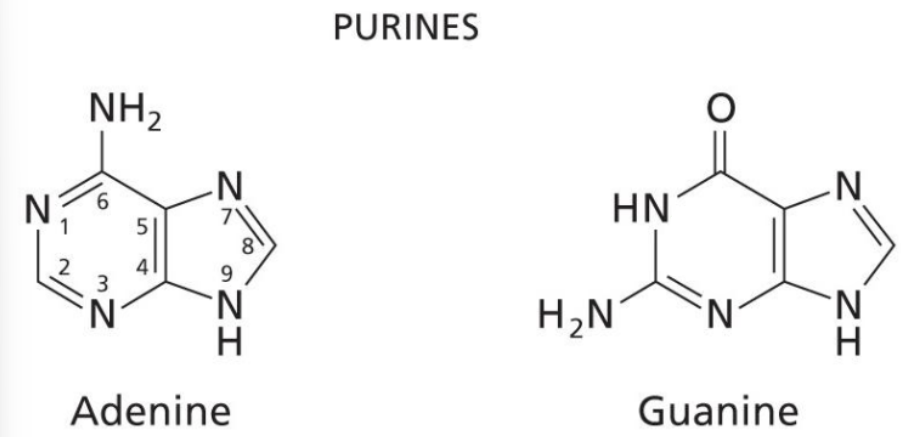
Purines
Smaller word larger molecule.
There are 4 N’s in the molecule.
There are no oxygens in adenine.
All bases are aromatic and heterocyclic.
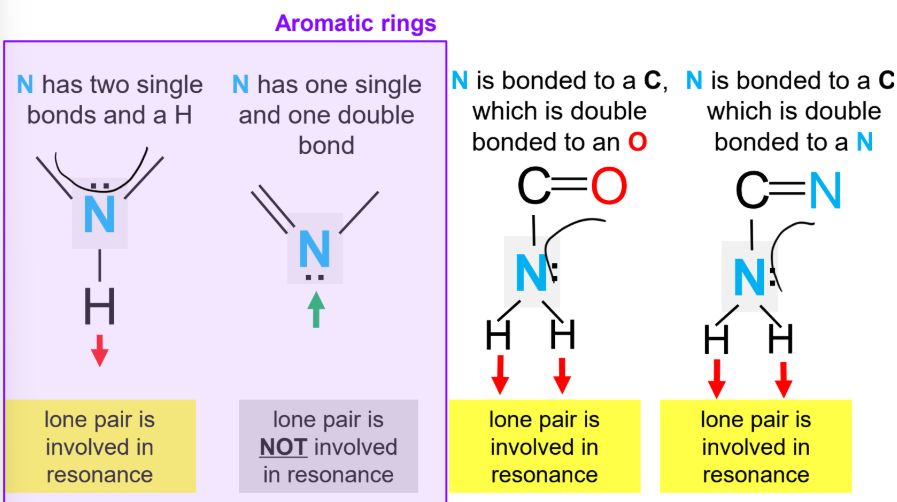
Resonance
Describes delocalized electrons when bonding cannot be shown by a single Lewis structure.
Occurs when electrons flow through neighboring π-systems; involves π-electrons or certain lone pairs.
Delocalization stabilizes molecules but reduces H-bonding capacity; resonance lone pairs cannot accept H-bonds.
Lone Pairs in Resonance & H-Bonding
Lone pairs involved in resonance cannot accept hydrogen bonds.
In aromatic rings, an N with two single bonds and an H has its lone pair involved in resonance, while an N with one single and one double bond has a lone pair not involved in resonance.
When N is bonded to a C that is double bonded to O, the N lone pair is involved in resonance.
When N is bonded to a C that is double bonded to N, the N lone pair is involved in resonance.
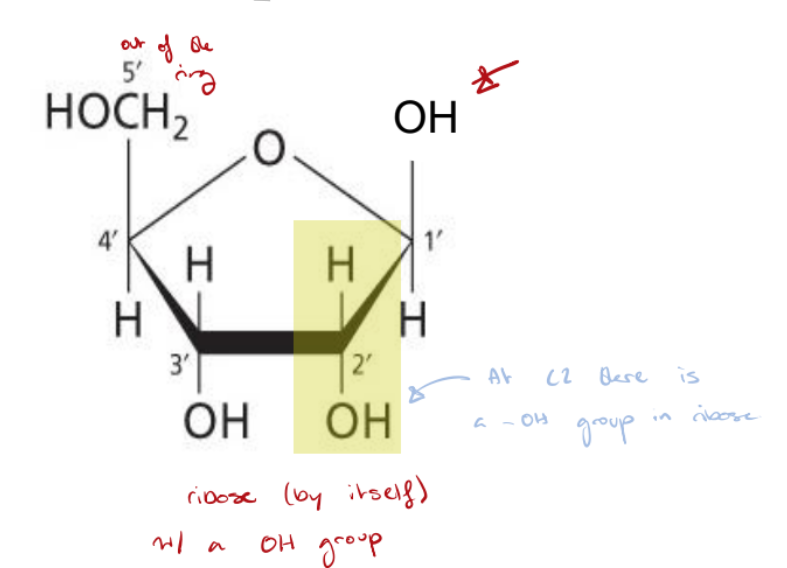
Ribonucleic Acid (RNA)
Polymer of G, A, C, U.
Sugar portion is ribose + heterocyclic.
C5’ is the only carbon atom not part of the ring structure.
At C2 there’s an -OH group.
Deoxyribonucleic Acid (DNA)
Polymer of G, A, C, T.
Sugar potion is deoxyribose.
There is no OH at the C2 position - literally lacking a oxygen.