PChem Exam 3
1/183
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
184 Terms
what are the separation methods
solvent extraction
chromatography
spectroscopy
types of chromatography
HPLC
gas chromatography
mass spectrometry
thin layer chromatography
how to calculate the partition coefficient
K=C1/C2
K=g compounds per mL of organic solvent/water
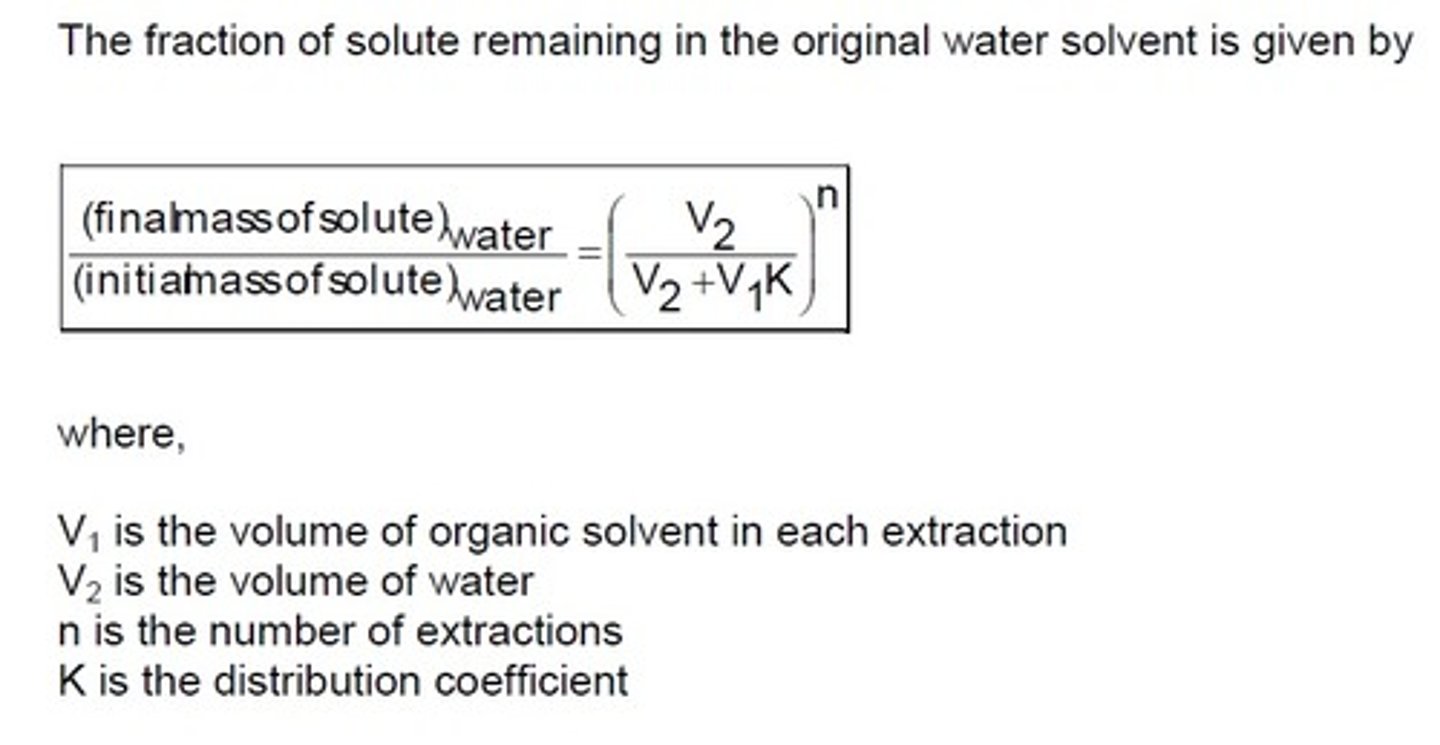
how to calculate the fraction remaining in water
final/initial = v2/(v2+v1K)^n
v1= vol of organic solvent
v2= og vol of water
how to make solvent extraction more efficent
do more extractions (bigger n)
more solvent comes out with each extraction
what molecules go into the aqueous layer
charged species, anything ionized
ex: water
what molecules go into the organic layer
neutral species, not ionized
ex: ethyl
to extract an acid into water, pH must be
higher than pKa
to extract a base into water, pH must be
lower than pKa
pH
becomes ionized (charged)
gains H
pH>pKa
does not become ionized
loses H
what does NaOH do
deprotonates
takes away H
usually from OH
this makes it charged so it goes into the aqueous layer
what does HCl do
protonates
adds an H
usually on NH2s (amines)
this makes it charged so it goes into the aqueous layer
what can HCl not protonate
amides (NH)
extraction with a metal chelator (aqueous and organic layers)
metal chelators are weak acids
aqueous phase: metal ion (M^n+)
organic phase: metal ligand (MLn)
extraction with a metal chelator what can occur
modifying pH can bering a metal into either phase
if the pH is high, it will be extracted into the organic
if pH is low, it will remain in aqueous
what is chromatographic separation based on
polarity
functional group
size
boiling point
what is a stationary phase and common examples
immobile phase that compound and mobile phase moves over
SiO2, C8, C18
what is a mobile phase and common examples
a solvent or gas that moves over the stationary phase carrying the compound of interest
water/methanol, hexane/isopropanol
if the stationary phase is SiO2, how do molecules move
SiO2 is very polar
polar molecules interact with SiO2 so those will moves slower
parameters of chromatography that can be manipulated
stationary/mobile phase composition
flow rate of mobile phase
size of stationary particle
level of packing and amount of stationary phase
height and width of column
what is band broadening and why do we want to avoid it
when peaks become thick and wide
more broad = less efficient column
things that can cause band broadening (refers to what occurs on the graph)
longitudinal diffusion (refers to the movement)
long retention time (too long on column)
particle size (bigger particle makes it hard to move through the column)
flow rate (too faster causes less separation)
injection time
column length (longer column inc retention time)
eddy diffusion (different paths taken through column, some may be longer than others, effects retention time)
amount of compound (more compound more broadening)
poor set up
ways to reduce longitudinal diffusion
faster flow rate
shorter column
smaller particles
open tubular columns
column is lined with the stationary phase which results in
high resolution
increased sensitivity
short analysis times
small sample capacity
packed columns
column is packed with tiny particles lined with stationary phase which leads to
low resistance
high flow rate
can make column longer to give more plates which increases efficiency
ideal resolution and what can effect it
>1.5
can be effected by
column length (longer column better resolution but not too long are can cause too much band broadening)
stationary phase
mobile phase
temperature GC
ideal asymmetry factor
AF=b/a=1
causes for fronting (AF less than 1)
overloading (too much compound, interactions are limited)
poor trapping (molecule doesn't interact well with solid phase)
injection solvent too strong (HPLC)
causes for tailing (AF greater than 1)
absorption to column (binds too well, hard to get off)
too much dead volume
ion exchange chromatography
separates based on the charge
stationary phase can be cationic/anionic
if made up of cations (+), anions (-) will be attracted and + ions pass through
anion exchange resin in ion exchange chromatography
only anions are attracted to it
molecular/size exclusion chromatography
separates based on size
small molecules go through pores (slow)
large molecules are excluded and go around (fast)
affinity chromatography
separates based on affinity
stationary phase is made up of things that attract other things (ligand)
attracts what has an affinity to it (ligand of interest)
if not attracted, then molecule will just wash through
area under the curve (AUC) is
proportional to the concentration
peaks require both
AUC and calibration curve
** peak must go back down to baseline for an accurate AUC
thin layer chromatography
separates by polarity to identify compounds by comparing to standards
stationary phase: SiO2 (polar)
mobile phase: hexane (nonpolar) /ethyl acetate (polar)
how do molecules travel on thin layer chromatography
stationary phase is POLAR
NONpolar molecules travel farther (top)
POLAR molecules do not travel as much (bottom)
silica holds polar closer, nonpolar doesnt interact and goes up
what happens to molecule if there is more hexane (NP) than ethyl acetate in the solvent (mobile phase)
hexane is nonpolar
more nonpolar, less interactions with silica, moves uo
ex: 30% hexane, 50% hexane, 70% hexane
70% hexane will have spots that are furthest down compared to 30% hexane which is closer to silica
what would you do if you want to increase the separation of a TLC plate
increase NONpolar solvent (hexanes) only when using SiO2 as a stationary
nonpolar will separate more bc it doesn't interact with SIO2
what is HPLC
High Performance Liquid Chromatography
form of column chromatography
pumps a sample mixture or analyte in a solvent (mobile phase) at high pressure through a column with chromatographic packing material (stationary phase)
parameters that can be manipulated in HPLC
stationary/mobile phase composition
flow rate of mobile phase
size of stationary particle
level of packing and amount of stationary phase
height and width of column
what limits HPLC
pressure and time
if you decrease particle size, what happens
efficiency increases
column/plate height decreases
plate number increases
pressure increases
why do you want a smaller particle size
allows for more interaction
it gives the sample more opportunity to bump into the particles to go through the stationary or mobile phase
what are common polar phases
amino, cyano, diol
what are common nonpolar phases
C18, C8, C4, phenyl
what are some stationary phase compositions
C18, C8, SiO2
what do you need for a mobile phase
2 solvents with differing polarities
(nonpolar and polar)
reverse phase (stationary phase)
C18
reverse phase (mobile phase)
polar: water
nonpolar: methanol, acetonitrile
normal phase (mobile phase)
polar: methanol, isopropanol
nonpolar: hexane
normal phase (stationary phase)
SiO2
isocratic elution does what to concentration
holds concentration constant (same throughout)
what is isocratic elution used for
used to separate early OR late peaks
cons of isocratic elution
easy separations can cause long elution times
gradient elution does what to concentration
varies the concentration by changing the mobile phase solvents/gradients
what is gradient elution used for
used to separate BOTH early AND late peaks
what is the relationship between pressure and solvent
the more viscous (thick) the solvent is the higher the pressure on the system
water vs MeCN viscosity
water is more viscous than MeCN so it causes more pressure
MeCN is less viscous than water so it causes less pressure
compound on a C18 column (reverse or normal phase)
reverse phase
how would compounds elute on a C18 column with water/methanol as a mobile phase
mobile phase is water/methanol which is nonpolar
most POLAR molecule will elute FIRST because it has the most interactions with the NONpolar mobile phase
how would you increase the resolution of separation on a C18 column with water/methanol as a mobile phase
normal phase
decrease particle size to increase efficiency
increase water concentration
(C18 is nonpolar, water is polar, more separation of polar compounds)
how would you increase the resolution of separation on a SiO2 column with hexane/isopropanol as a mobile phase
reverse phase
decrease particle size to increase efficiency
increase hexane concentration
(SiO2 is polar, hexane is nonpolar, more separation of nonpolar compounds)
why would you increase the water concentration to increase resolution of separation on a C18 column with water/methanol as a mobile phase
bad resolution or separation occurs due to fast retention time
if you decrease the retention time, the compounds would have more time to interact with the mobile phase
mobile phase is water/methanol which is nonpolar
if you increase water, you decrease polarity which allows the compounds to interact more with the nonpolar molecules since there isn't as many of them
this causes a longer retention time which results in better separation
what would happen if you increase the methanol concentration instead
too much nonpolar compounds to react which make the retention time super fast which results in bad resolution and low separation
compound on a SiO2 column (reverse or normal phase)
normal phase
how would compounds elute on a SiO2 column with hexane/isopropanol as a mobile phase
mobile phase is hexane/isopropanol which is polar
most NONpolar molecule will elute FIRST because it has the most interactions with the POLAR mobile phase
what moves faster in a reverse phase
polar moves faster
what moves faster in a normal phase
nonpolar moves faster
how does pH effect the eluent
effects retention time, separation, and resolution
how can you manipulate the pH of the eluent
used in reverse phase
uses buffers (salt mixtures) at various pH to change composition
when pH is near the pKa of the compound what happens to resolution/separation
compounds do not have bad resolution because they do not move or elute as much
when pH is not close to the pKa of the compound what happens to resolution/separation
compounds have better separation because they move/elute more
how does temperature effect HPLC
if you increase temperature
capacity factor decreases
retention time decrease
peak efficiency increase
elution time decreases
selectivity decreases
what is a molecular ion
M+ or M+H
doesn't always have to be the largest peak, sometimes does not show up
components of an ideal detector
sensitive and selective
universal
provide linear response
not broaden peaks
application of HPLC with an external standard
1. run chromatograms with varying concentrations of the analyte (crush a pill and run it on HPLC)
2. generate a calibration curve to determine concentration and amount (use pure standards at different concentrations)
3. run the sample to determine the concentration and compare to standard
**essentially comparing the standard to your unknown to determine what you have and how much you have
application of HPLC with an internal standard
choose an internal standard that is
close in structure to the elute (creates a similar detector response)
stable and pure
make sure you have a known amount
then you run it through a chromatograph and use the area under the curve and compare concentrations
**inject a similar compound (betamethasone) to the compound (hydrocortisone) to determine how much you have
requirements of gas chromatography
molecules must be small and volatile
column needs to be long BUT
flow rate needs to be high
mobile phase of gas chromatography
carrier gases such as He, N2, H2
stationary phase of gas chromatography
SiO2
how does gas chromatography work
analyte is injected into a heart port where it evaporates and then moves through the column by a carrier gas (He, N2, H2)
parameters of capillary gas chromatography
carrier gas flow (needs to be high)
carrier gas type (He, H2, N2)
column temp (lower temp for better resolution)
column length (longer length for lower temp for better resolution)
column diameter (smaller is better)
types of gas chromatography detectors
flame ionization
mass spectrometry
types of mass spectrometry
magnetic sector
single/triple quadrupole
time of flight (TOF)
ion trap
magnetic sector
ions accelerated by magnet where only certain ions can get through (depends on voltage)
small goes up (top)
heavy goes down (bot)
single quadruple
ions that resonate with the frequency (radio wave) applied can get through
**certain freq give certain ion
triple quadruple
uses 2 quadrupoles that can select for mass (thus 2 mass separations)
hits ions with collision gas which causes FRAGMENTATION
time of flight (TOF)
ions get accelerated
small ions travel faster but HIGH ENERGY travels first
reflectron opposes direction of high energy ion delaying the time (why small ions reach detector first even though high energy travels first)
VERY SENSITIVE
ion trap
ions injected into a trap and start oscillating at a certain frequency
VERY SENSITIVE
what happens when you combine TOF and ion trap
since both are very sensitive, you can get up to 4 decimal places which helps determine molecular formula
ionization methods
electron impact
chemical ionization
electron spray ionization
atomic pressure chemical ionization
MALDI
DART?
electron impact
molecule is hit with very high energy
CAUSES FRAGMENTATION
chemical ionization
ionizes molecule with methane (a chemical instead of energy like electron impact)
similar to electron impact just not as high energy
lets you see parent ions molecular weight
electronspray ionization (ESI)
+ current
- ions attracted
+ions pass through
atomic pressure chemical ionization
used for nonpolar molecules
eluent passes through heated tube which forms an aerosol in which voltage is applied to generate ions
CAUSES FRAGMENTATION
MALDI (matrix associated laser deposition ionization)
used for large molecules (proteins or polymers)
sample gets blasted with laser where ion forms
DART (direct analysis in real time)
plasma contains either + or - and neutral atoms which water with water and become charged
types of HPLC detectors
ultraviolet
refractive index
evaporative light-scattering
fluorescence
mass spectrometry
fourier transform infrared