Acids and Bases
0.0(0)
Card Sorting
1/6
Earn XP
Description and Tags
Last updated 6:18 PM on 3/13/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
7 Terms
1
New cards
Organic **Basic** groups
Has lone pairs (RO- ; RNH2)
\
\
\*Since a base must accept an H+ , **BASES** in organic chemistry have **lone pairs** to accept the H+
\
\
\*Since a base must accept an H+ , **BASES** in organic chemistry have **lone pairs** to accept the H+
2
New cards
Organic **Acidic** Group
Has H attached to O/electronegative atom (ROH ; R-CO2-H)
\
\*Since an %%**acid must have an H+ available to donate**%%, acidic molecules in organic chemistry usually %%**have an H attached to an electronegative atom**%%
\
\*Since an %%**acid must have an H+ available to donate**%%, acidic molecules in organic chemistry usually %%**have an H attached to an electronegative atom**%%
3
New cards
Acid strength (type of bond)
triple bond > double bond > single bond
4
New cards
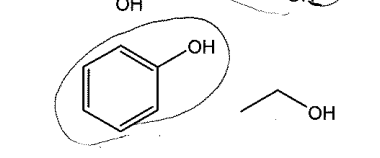
Acid strength (polarity)
nonpolar doesn’t equal bad acid (see pic)
5
New cards
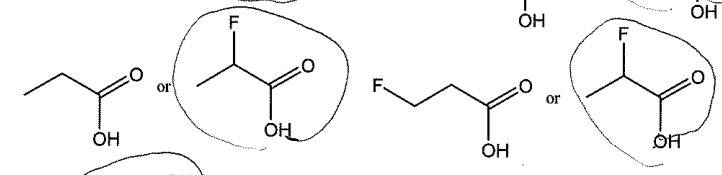
Acid Strength (electronegativity)
The more spread apart the electrons, the better
6
New cards
Acid Strength (atom size)
F>Cl>Br>I (opposite from SN2 NU!!)
7
New cards
Acid Strength (pKa)
the **higher pKa** the **worse** the acid