AP Chemistry Unit 2
1/36
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
37 Terms
Ionic Bonds
when one atom takes an electron from another (metal + nonmetal)
Covalent Bonds
the equal (or unequal) sharing of electrons between atoms (nonmetal + nonmetal)
Metallic Bonds
when neither atoms have a strong attraction to an electron (metal + metal)
Metallic Substances
held by metallic bonds, good conductors of electricity/heat, don’t dissolve, malleable/ductile
Molecular Compounds
do not conduct electricity, some of them dissolve
Ionic Solids
held by ionic bonds, very high melting/boiling point, don’t conduct electricity as solids but do when dissolved/melted
Covalent-Network Solids
held by covalent bonds, high melting/boiling points, don’t dissolve, don’t conduct electricity
Lattice Energy
energy required to separate one mole of ionic compounds
Larger Atomic Radius
decreases lattice energy
Bond Order
number of bonds between a pair of atoms
Higher Bond Order
shorter bond length due to higher electrostatic attraction
Formal Charge Formula
valence electrons - # of bonds - # each electron around atom
Alloys
a mixture of one or more elements with metallic properties
Substitutional Alloy
formed by elements with a similar atomic radii
Interstitual Alloy
formed by elements w/different atomic radii
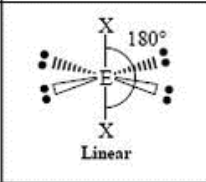
2 Domain, 0 LP
linear

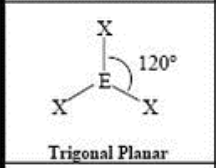
3 Domain, 0 LP
trigonal planar (120)
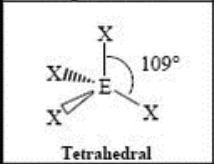
4 Domain, 0 LP
tetrahydral (109.5)
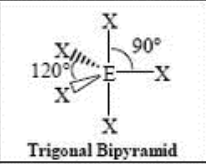
5 Domain, 0 LP
trigonal bipyramidal (120/90)
6 Domain, 0 LP
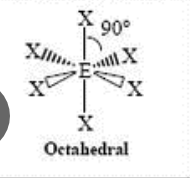
octahedral (90)
Sigma Bonds
formed by head-to-head overlap of orbitals (stronger)
Pi Bonds
formed by side-by-side overlap of orbitals (weaker)
Hybrid Orbitals Order
sp, sp^2, sp^3 sp^3d, sp^3d^2, etc
Dipole Atoms
atoms with equal but opposite charges
Nonpolar Covalent Electronegativity Difference
0.0-0.04
Polar Covalent Electronegativity Difference
0.4-2.1
Ionic Electronegativity Difference
>1.7
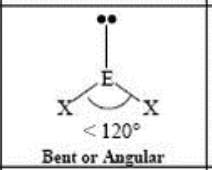
3 Domain, 1 LP
bent
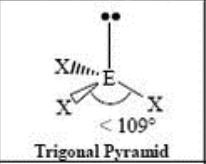
4 Domain, 1 LP
trigonal pyramidal
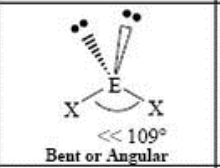
4 Domain, 2 LP
bent
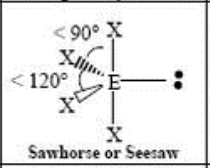
5 Domain, 1 LP
seesaw
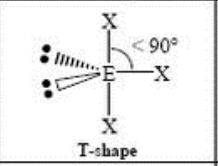
5 Domain, 2 LP
t-shaped
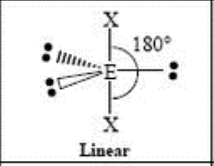
5 Domain, 3 LP
linear
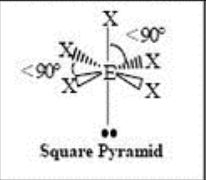
6 Domain, 1 LP
square pyramidal
6 Domain, 2 LP
square planar
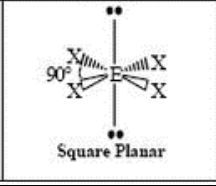
6 Domain, 3 LP
t-shaped
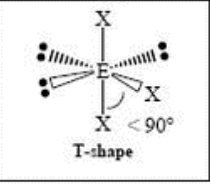
6 Domain, 4 LP
linear