Chapter 18 Ketones and Aldehydes
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
Carbonyl Compounds
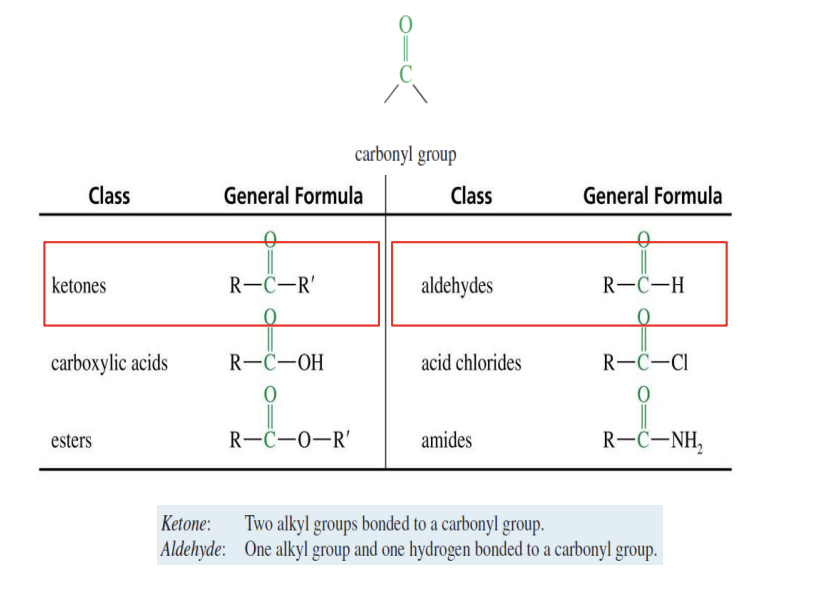
Ketone: 2 R groups (alkyl groups)
Aldehyde: 1 R group (alkyl group)
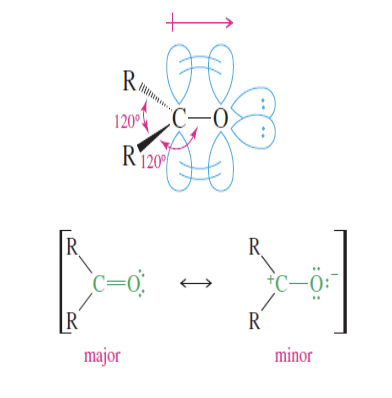
Carbonyl Strucutre
Main things to know is that the carbon is Sp2 hybridized, the C=O bond is polar with the O being more electronegative than the C
carbon given partial pos charge with resonance structures
Ketone Nomenclature
Alkane —> Alkanone
butane —> buta-#-one
the # is where the cabron the ketone’s oxygen is attached to
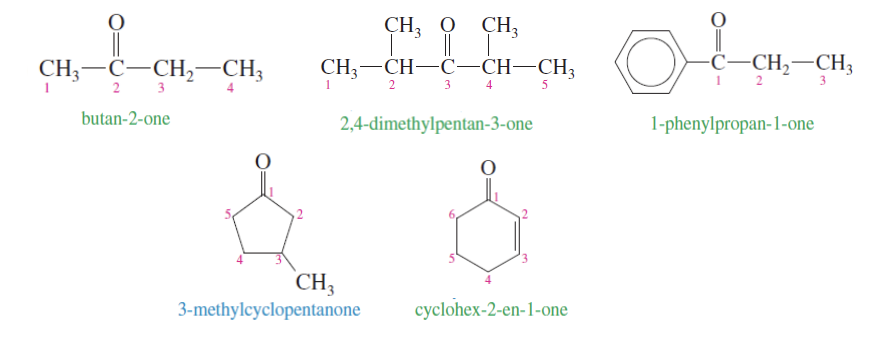
Aldehyde Nomenclature
Alkane —> Alkanal
numbering done based on the carbonyl carbon closest to the end
the aldehyde group is given the number 1
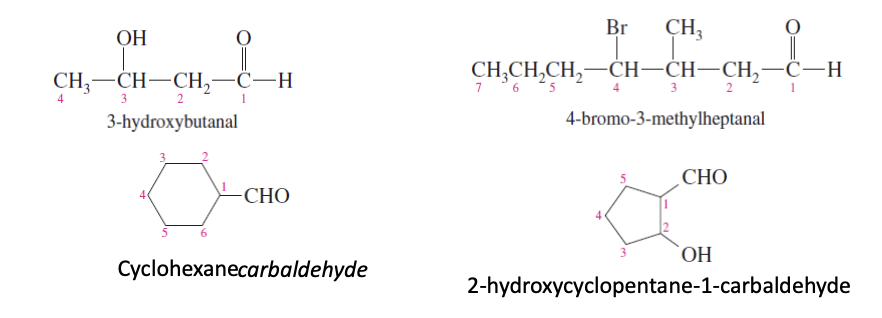
Ketone Substituents Nomenclature
If part of the long carbon chain —> writen as oxo group (3-oxopentanal)
If not part of the longest carbon chain —> writen as oyl group (2-pentanoylbenzoic acid)
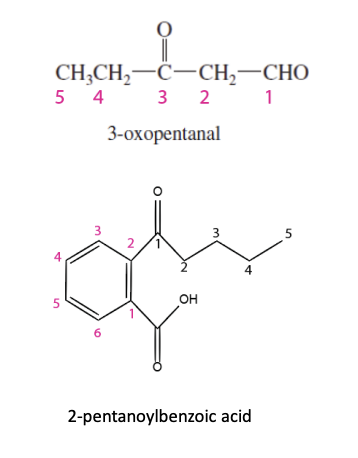
Aldehyde Substituents Nomenclature
If part of the longest chain —> oxo group
If not part of the longest chain —> formyl group
Boiling Points of ketones and aldehydes
More polar than alkanes and ethers —> higher bp than them
Can’t form H-Bonds to each other —> Lower bp than alcohol
BP ranking order: Alcohol > Ketone & Aldehydes > Esters > Alkanes
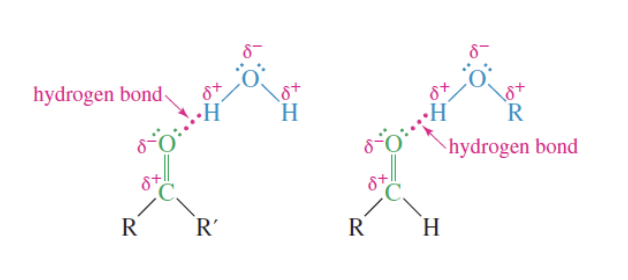
Solubility of Ketones and Aldehydes
Good solvent for alcohols
dissolve well in water —> oxygen’s lone pair of electrons can form hydrogen bonds with O-H or N-H
bonds with water
small ketones & aldehydes (acetone and acetaldehyde) are miscible are mix with water completely
Ketones & Aldehydes Syntheses
Alcohol has to be oxidized to become either ketone or aldehyde
With NaOCl/ HOAc —> Ketones
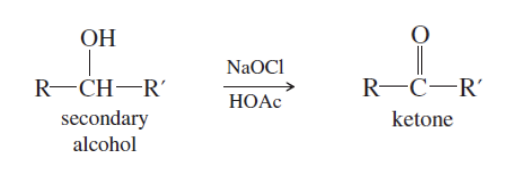
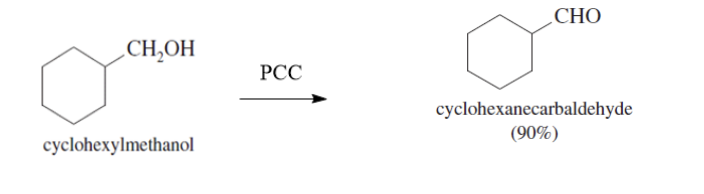
With PCC or NaOCl (TEMPO present) —> Aldehyde
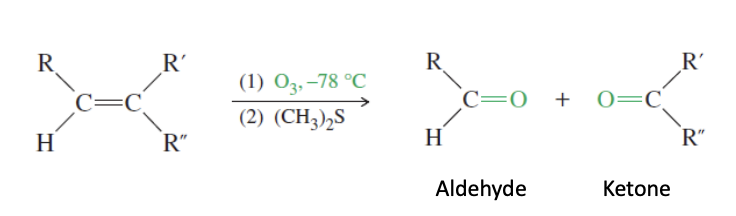
Alkene Ozonoloysis
Ozonolyisis: O3 used to form ketones and aldehydes from alkenes
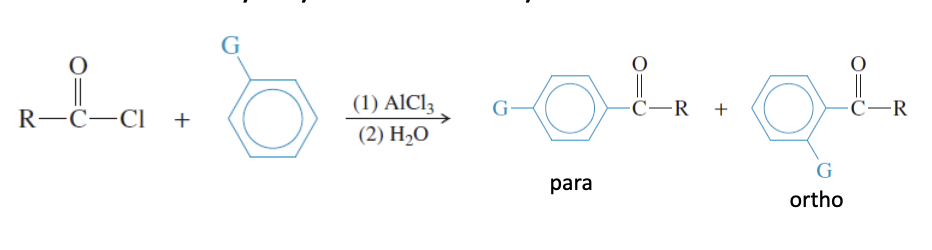
Friedel-Crafts Acylation
Aromatic Ring + Cl-Cl-ACl3 —> para ortho positions ketones