JEE Alcohols, Phenols and Ethers
1/88
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
89 Terms
carbinol
CH3OH
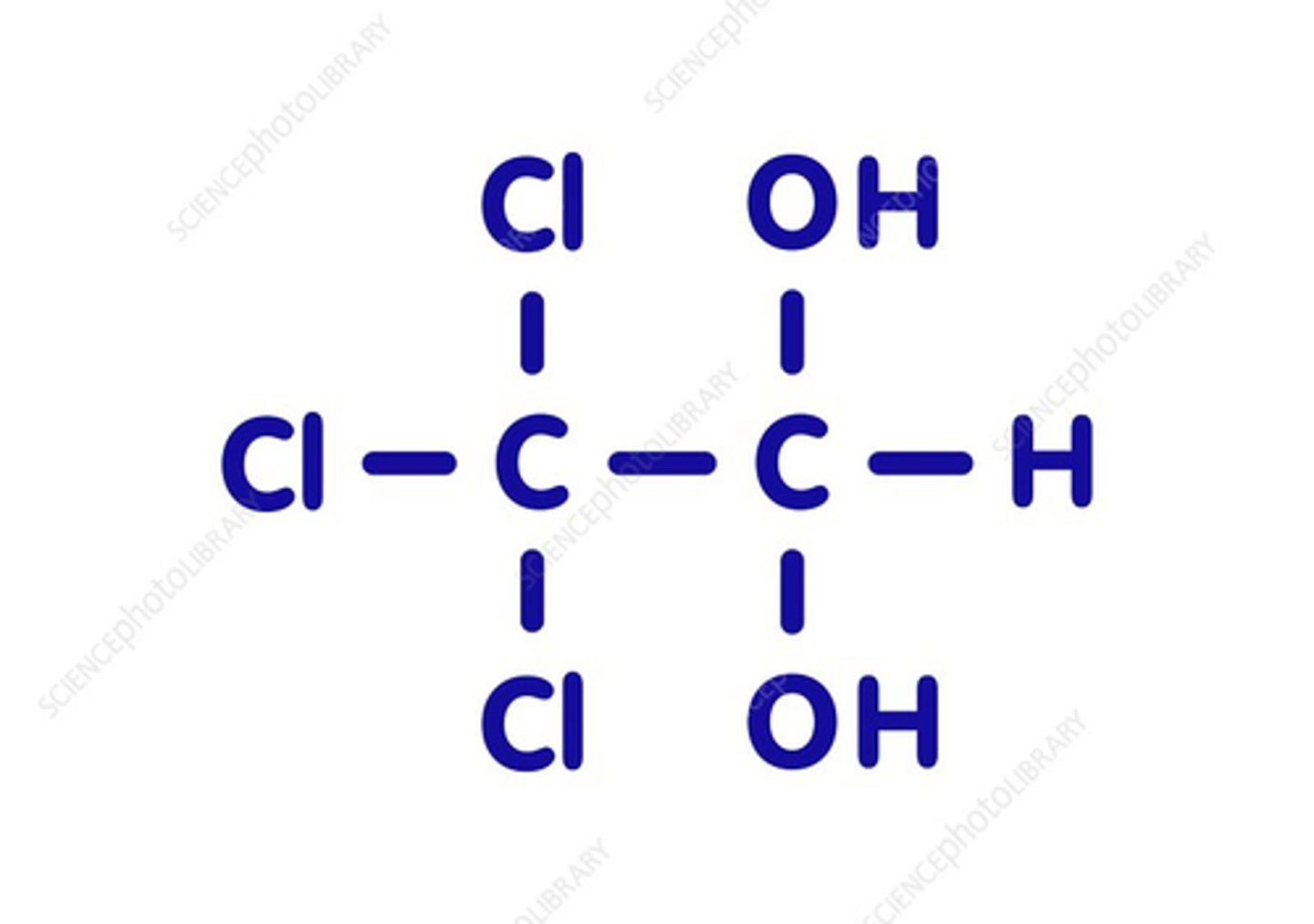
chloral hydrate
(stable)
action of Alk KMnO4 on 3⁰ alkane
3⁰ alcohol formed
Hydroboration reaction
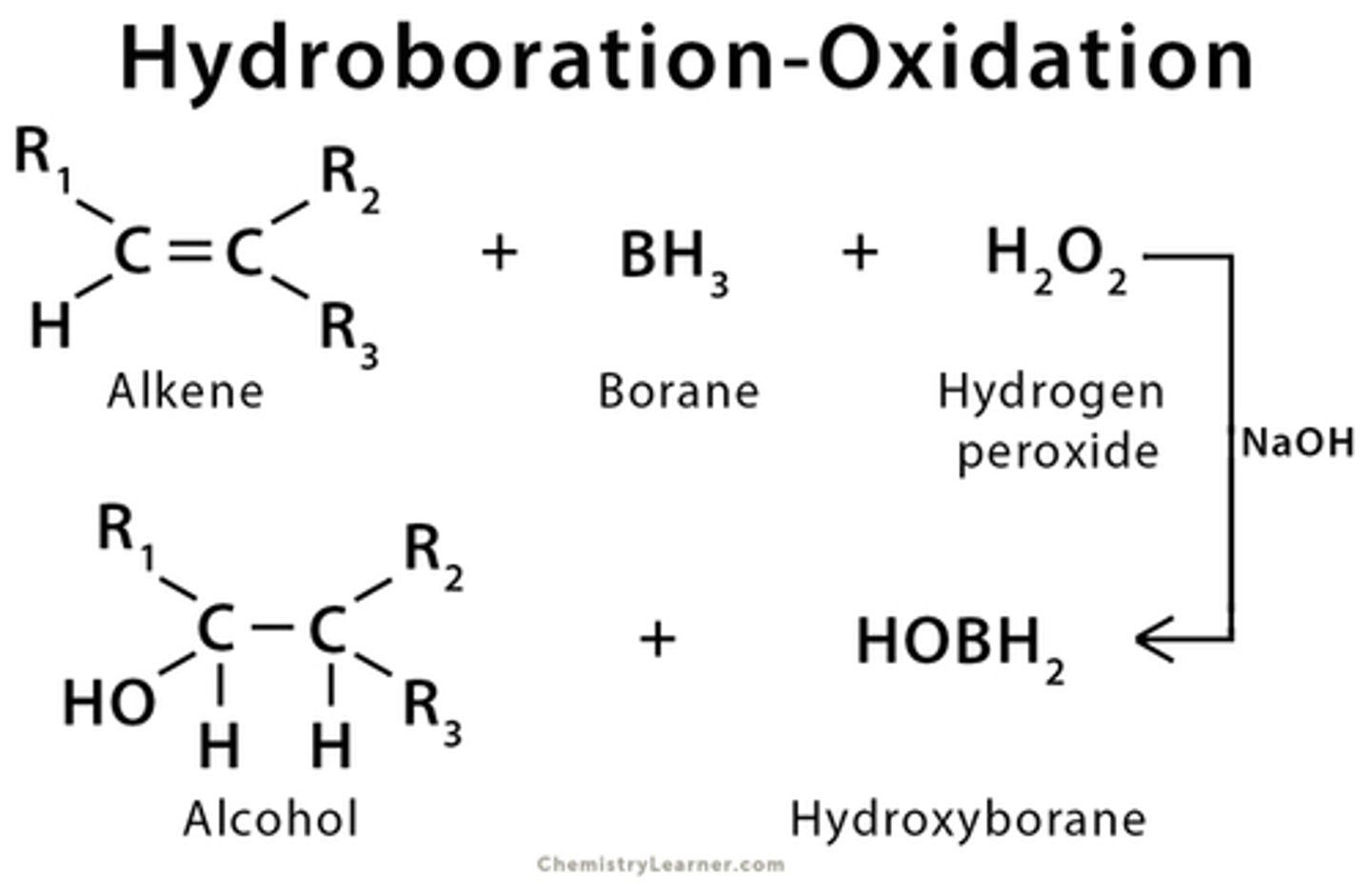
hydroboration oxidation forms _____ product(mark/antimark)
antimark
oxymercuration/demercuration forms
alcohols
hydroboration oxidation forms
alcohols
oxymercuration/demercuration reaction
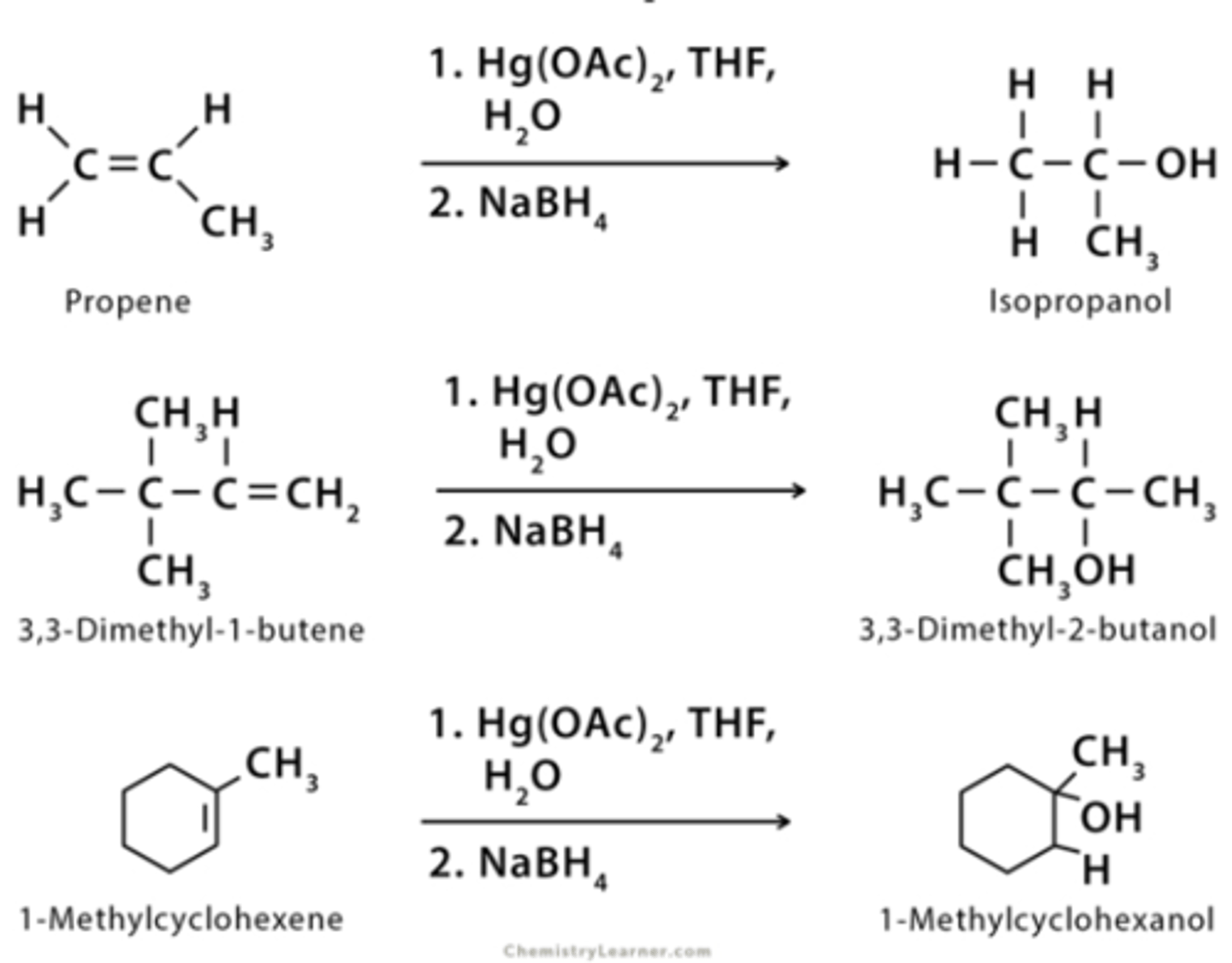
oxymerc/demerc reaction gives ___ product(markovnikov/antimark)
markovnikov
difference between products of hydroboration oxidation and oxymercuration/demercuration
Hydroboration forms antimark product, Oxymerc forms markovnikov product
Zn/HCl, Na/ethyl alc., LiAlH4 and NaBH4 are reducing agents. Which of the following cannot reduce =? What is the exceptional case where it can?
1) LiAlH4, NaBH4
2) LiAlH4 - when phenyl group is present at beta position
NaBH4 does not affect ___ in reduction
C=C, N=N, -COOH, ester, -CN-, NO
difference between action of HNO2/NaNO2/HCl on aniline and R-NH2
aniline - forms diazo salt(stable)
R-NH2 - forms diazonium ion, then alcohol
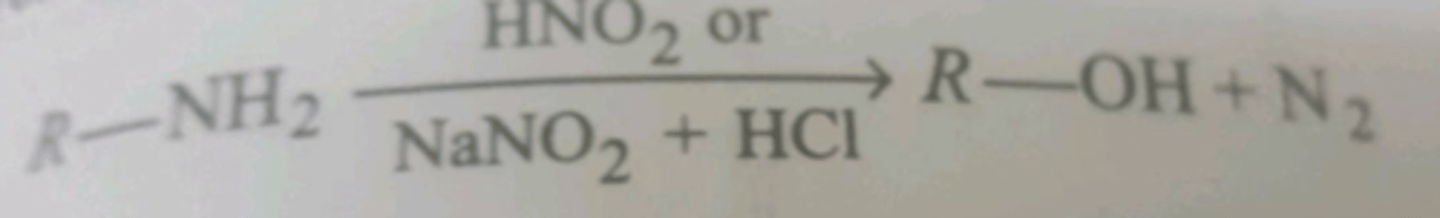
hydrolysis of ester yields
carboxylic acid + alcohol
which bond is cleaved in hydrolysis of ester?
C-O bond(not C=O bond)
invertase converts
glucose to fructose(GIF)
zymase converts
glucose + fructose to ethyl alcohol
diastase converts
starch to maltose
maltase converts
maltose to glucose
enzymes used in formation of ethyl alc from molasses
invertase, zymase
enzymes used in formation of ethyl alcohol from starch
diastase, maltase, invertase, zymase
Reactivity of alcohols where cleavage of O-H takes place is of order
primary > secondary > tertiary
reactivity of alcohols where cleavage of C-O takes place
tertiary > secondary > primary
order of acidity of alcohols
primary > secondary > tertiary
alcohols are ___ acids(strong/weak)
weak
ease of dehydration of alcohols is of order
tertiary > secondary > primary
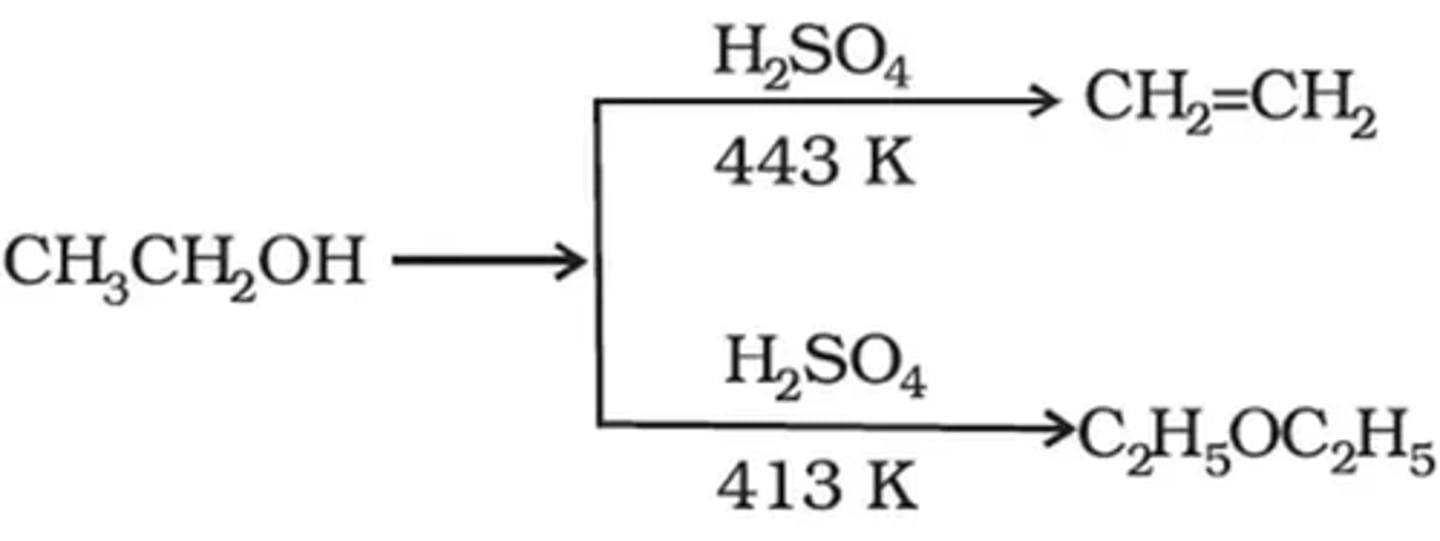
action of H2SO4 on C2H5 - OH
at 140°C - ether formed
at 180°C - alkene formed
action of Cu on R-CH2-OH
R-CHO
action of CAN on R-CH2-OH
R-CHO
MnO2 is used to oxidize ___ alcohols
benzylic, allylic
Action of Al(OCMe)3/CH3-O-CH3 on alcohols(R-CH2-OH)
R-CO-R + CH3-CH(OH)-CH3
Jones reagent
CrO3
action of CrO3 on alcohols
- primary alcohols converted to -COOH
- secondary alcohols converted to ketones
name the reagents that can replace CrO3 in Jone's reaction
KMnO4, Acid Dichromate, Aq Chromic acid, Collin's reagent, PDC/CH2Cl2
action of PDC/CH2Cl2 on alcohol
- primary alcohols converted to -COOH
- secondary alcohols converted to ketones
action of DMS/(COCl)2 on alcohols
- primary alcohols converted to -COOH + pyridine
- secondary alcohols converted to ketones
Haloform test comes positive for
2 degree alcohol, acetaldehyde, ethyl alcohol, methyl keto group
ROH + HClO4 is a test for alcohol. What is the colour of precipitate?
blue green, opaque
catalytic dehydrogenation of 3 degree alcohol gives
alkene
catalytic dehydrogenation of primary alcohol gives
aldehyde
catalytic dehydrogenation of secondary alcohol gives a
ketone
in Victor Meyer test, red colour indicates
1 degree alcohol
in Victor Meyer test, blue colour indicates
2 degree alcohol
in Victor Meyer test, colourless solution indicates
3 degree alcohol
___ synthesis forms ethers
Williamson
what is the combination of reagents for best yield for Williamson's synthesis
primary alkyl halide + 3 degree alkoxide
can Ph-O-Ph be formed using Williamson's synthesis?
no(if both R and R' are phenyl, tertiary, or vinyl then williamson's synthesis won't occur)
Can Ph-X + CH3-ONa be used for williamson's synthesis
phenyl group on halide cannot be used for Williamson's synthesis(Good yield is for primary alkyl halide and tertiary alkoxide)
Alkoxy-Mercuration Demercuration

difference between oxymerc/demerc and alkoxymerc/demercuration
oxymerc/demerc uses H2O, and gives alcohol
alkoxymerc/demerc uses ROH, and gives ether
Ether + H2SO4
alcohol(bond cleavage of ether)
ether + HI
alcohol and alkyl iodide(C2H5-O-C2H5 + conc. H2SO4 ----> C2H5I + C2H5OH, 2 moles of I gives 2C2H5I)
Ph-O-R + HX --->
RX + Ph-OH
ether + CO
ester
C2H5-O-C2H5 +Cl2 --->
(1) in the dark
(2) in the presence of light
(1) CH2(Cl)-CH(Cl)-O-CH(Cl)-CH2(Cl)
(2) C2Cl5-O-C2Cl5
C2H5-O-C2H5 + O2 --->(in BF3/150°C)
C2H5 - O - O - C2H5(ethyl propionate)
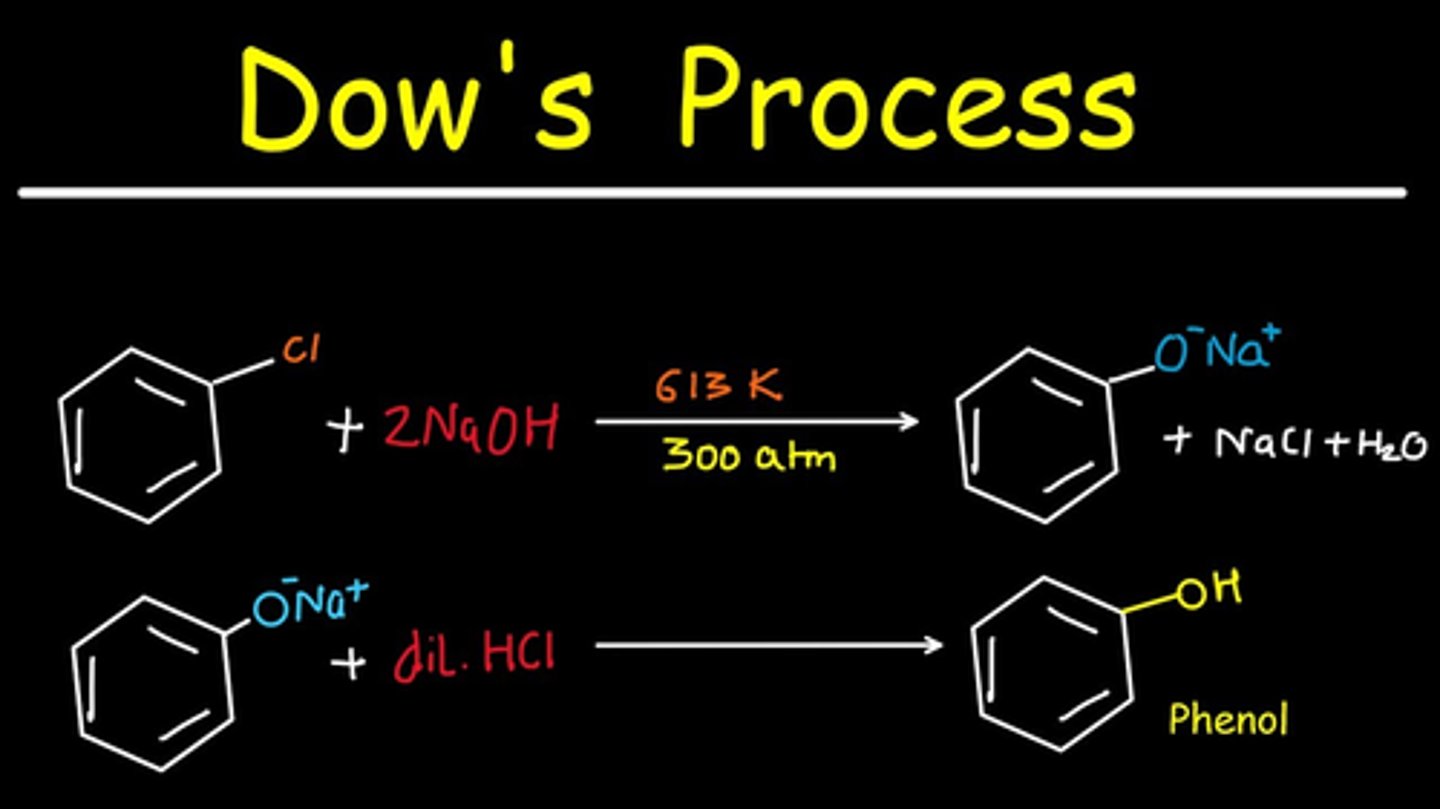
Dow's reaction gives ___ product
phenol
preparing phenol from Ph-SO3H
Ph-SO3H + NaOH -> Ph-ONa + HCl -> Ph-OH
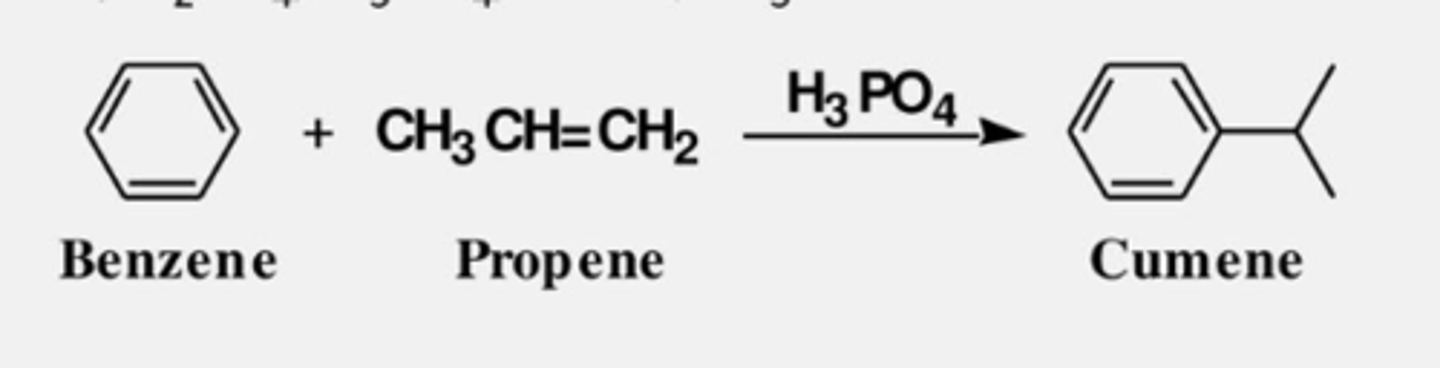
Benzene + CH2=CH-CH3 (H3PO4) --->
Cumene, which on oxidation gives phenol
Dalkin reaction acts on
o- or p- hydroxyaldehyde or o- or p- aminoaldehyde
action of H2O2 + NaOH on o- or p- hydroxyaldehyde
catechol

Dakin reactions results in
catechol
which are more acidic? Phenols or Alcohols? State why.
Phenols, due to high stability of Phenoxide ion
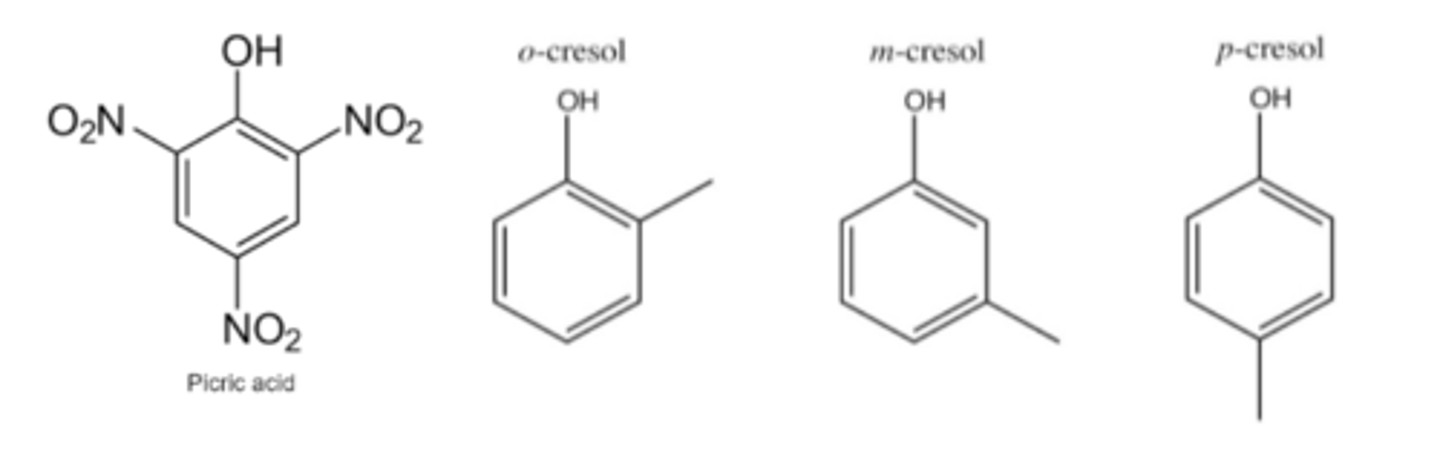
order of stability of
1) phenol
2) m-cresol
3) p-cresol
4) o cresol
5) m nitrophenol
6) p- nitrophenol
7) o-nitrophenol
8) 3, 5- dinitrophenol
9) picric acid
picric acid> 3,5-dinitrophenol > p-nitrophenol > o - nitrophenol > m-nitrophenol > phenol > m-cresol > p-cresol > o-cresol
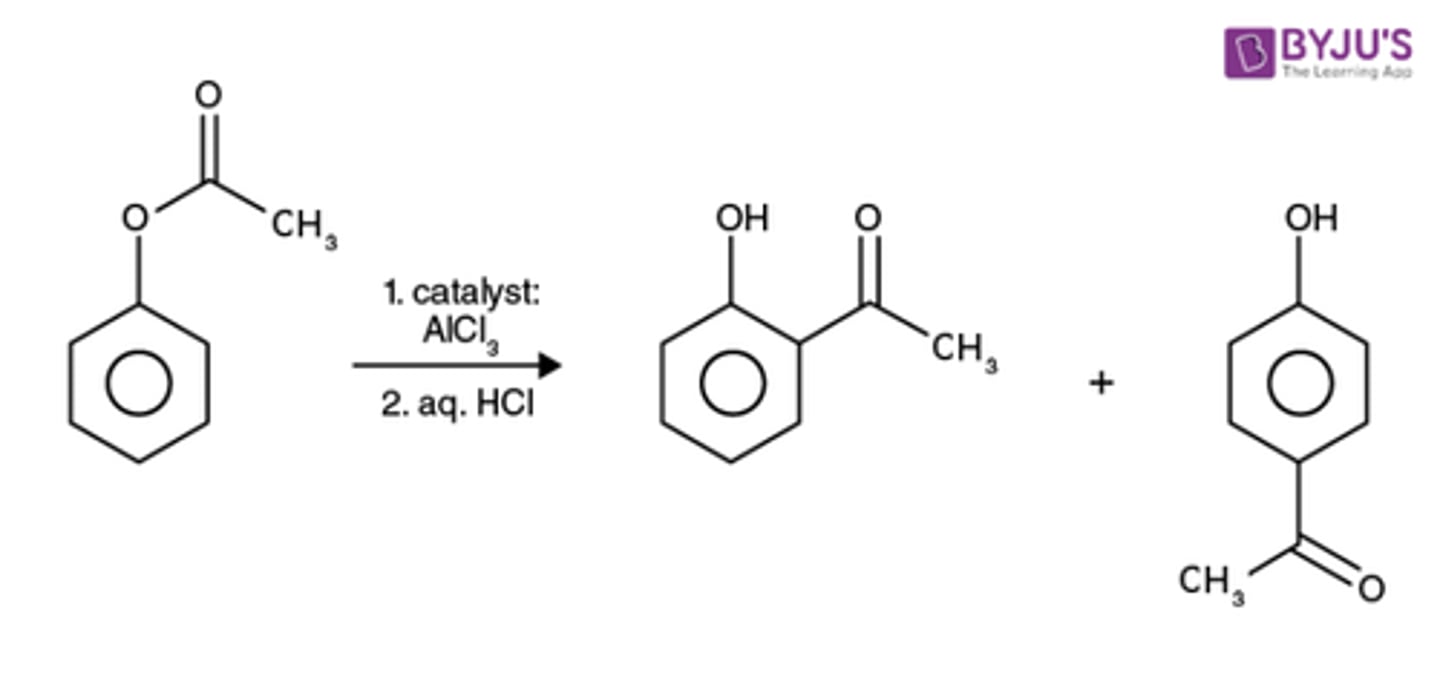
Schotten Bauman esterification
Ph-OH + RCOOH ---> Ph-O-CO-R + H2O
Ph-OH + RCOCl in pyridine gives
Ph-O-CO-R + HCl
acetylation of Salicylic acid gives
aspirin
reaction for preparation of aspirin
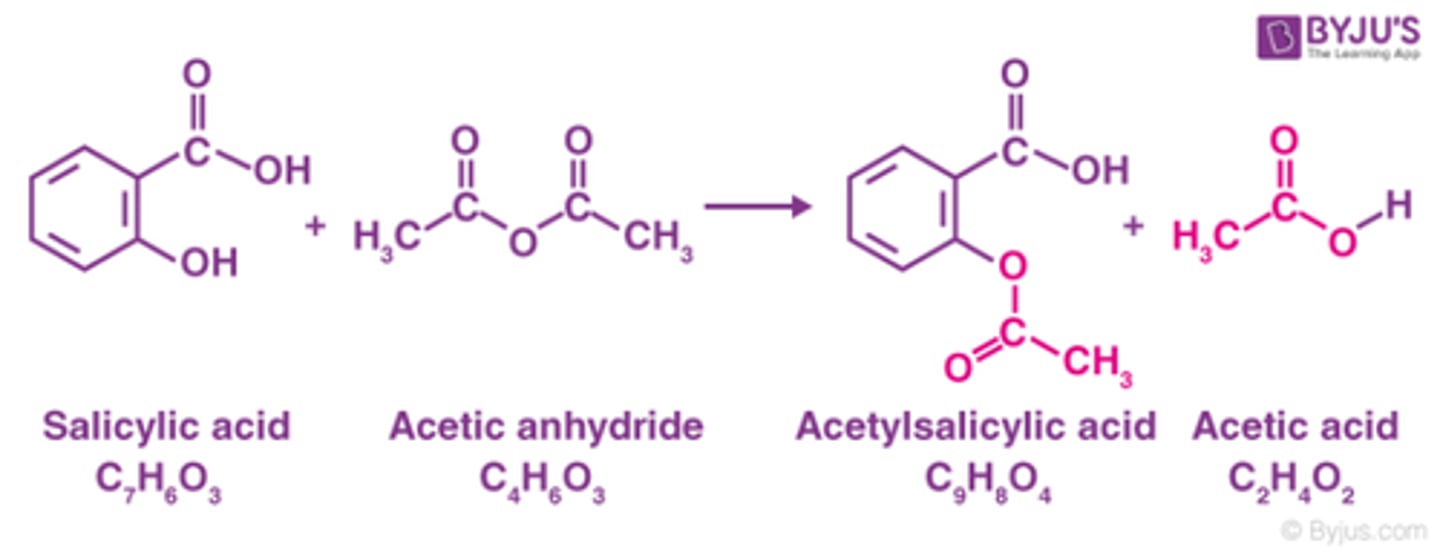
acetyl salicylic acid is also known as
aspirin
order of melting point for o-nitrophenol, p-nitrophenol and m-nitrophenol
p-nitrophenol>m-nitrophenol>o-nitrophenol (p nitrophenol has intermolecular hydrogen bonding, m does too, but o nitrophenol has intramolecular hydrogen bonding, which restricts its intermolecular hydrogen bonding)
Phenol + conc. HNO3
picric acid
Phenol + Br2 in CS2
o-bromo phenol + p bromo phenol
Phenol + Br2 in H2O
2, 4, 6 tribromo phenol
Kolbe's reaction product
salicylic acid
Kolbe's reaction reagents
NaOH, CO2, H2SO4
Kolbe's reaction

Riemer Tiemann reaction forms
o-hydroxy benzaldehyde
Riemer Tiemann reaction
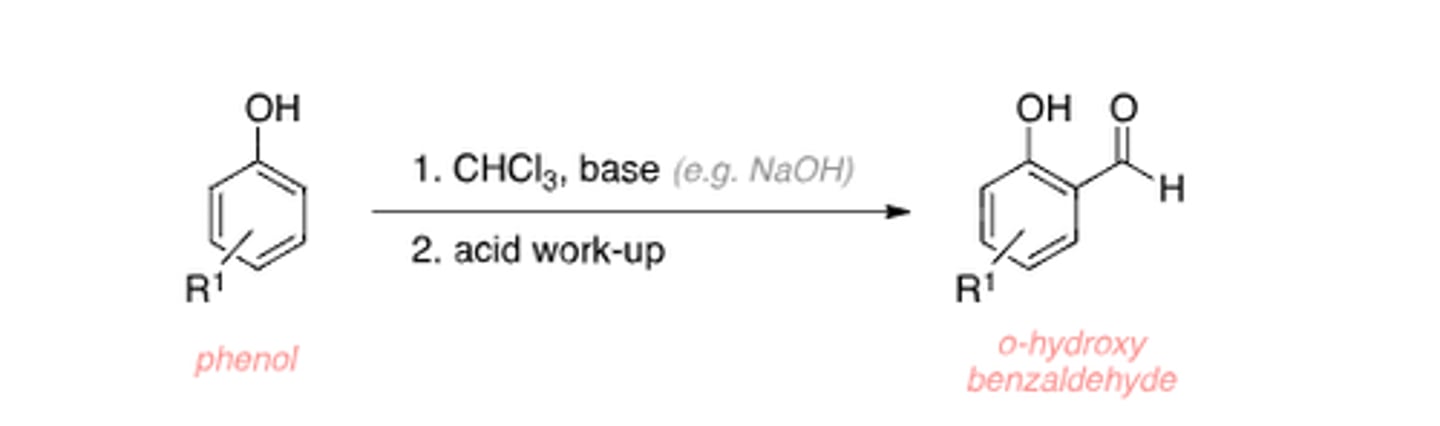
Riemer Tiemann reaction intermediate
:CCl2
if ortho position is occupied, Riemer Tiemann reaction product is
para - hydroxy benzaldehyde
if Potassium phenoxide is used instead of Sodium Phenoxide in Kolbe's reaction, what happens?
Para isomer of salicylic acid is formde
action of K2S2O7 on phenol
Elb's persulphate reaction
In libermann nitroso reaction, product formed is

Order of colours observed in Libermann nitroso reaction
blue green, to red, to deep blue
compound that gives deep blue colour in Libermann nitroso reaction
(K can be replaced with Na)
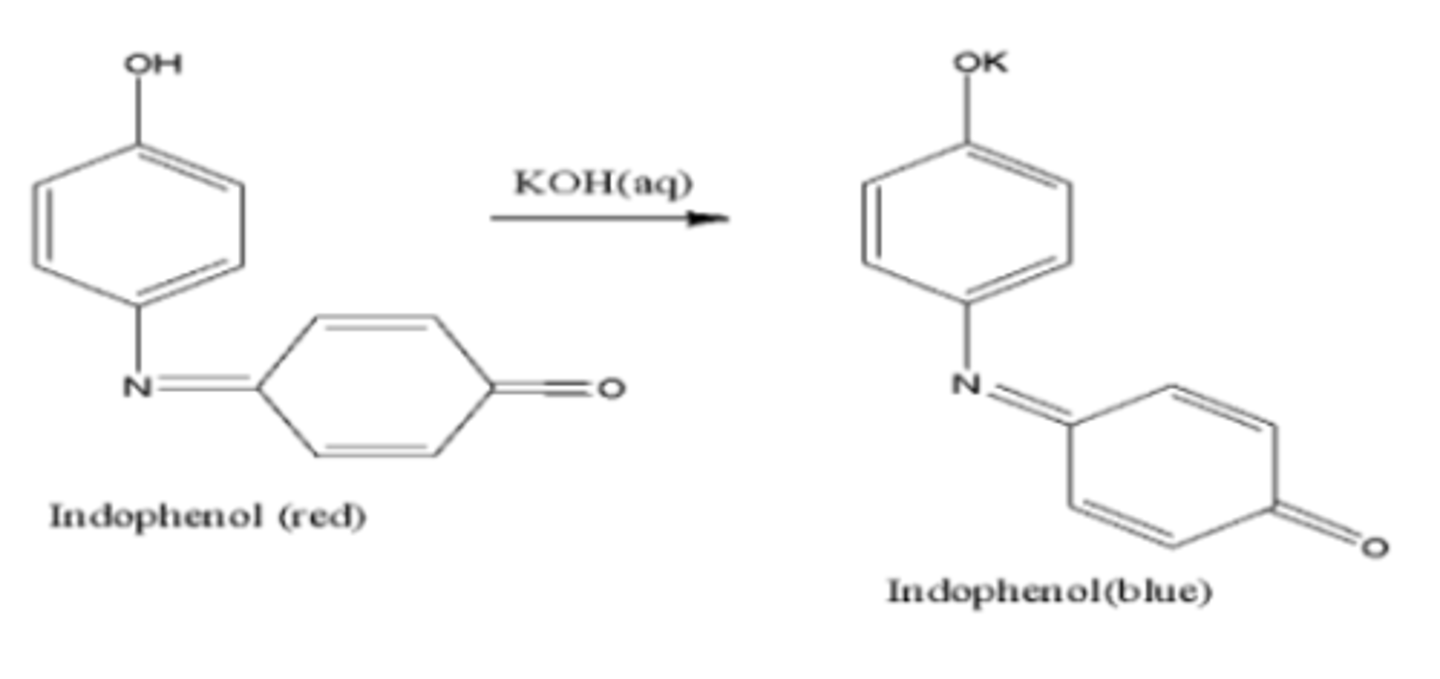
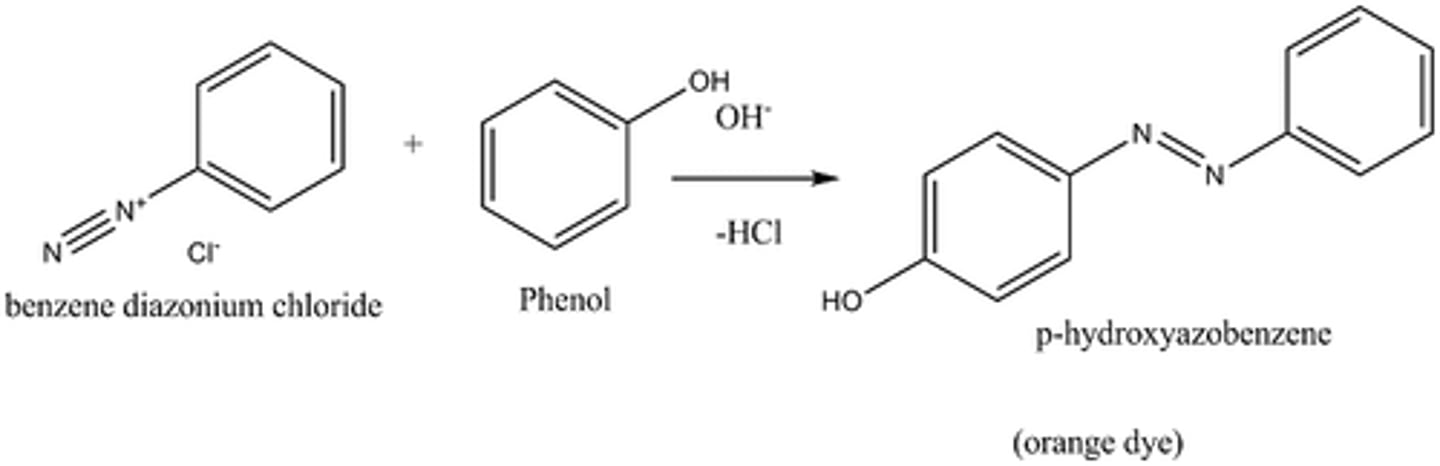
diazonium salt + phenol
(the -N=N- attaches itself at para or ortho position(if para not available) of -OH group)
action of Zn dust on phenol
forms benzene
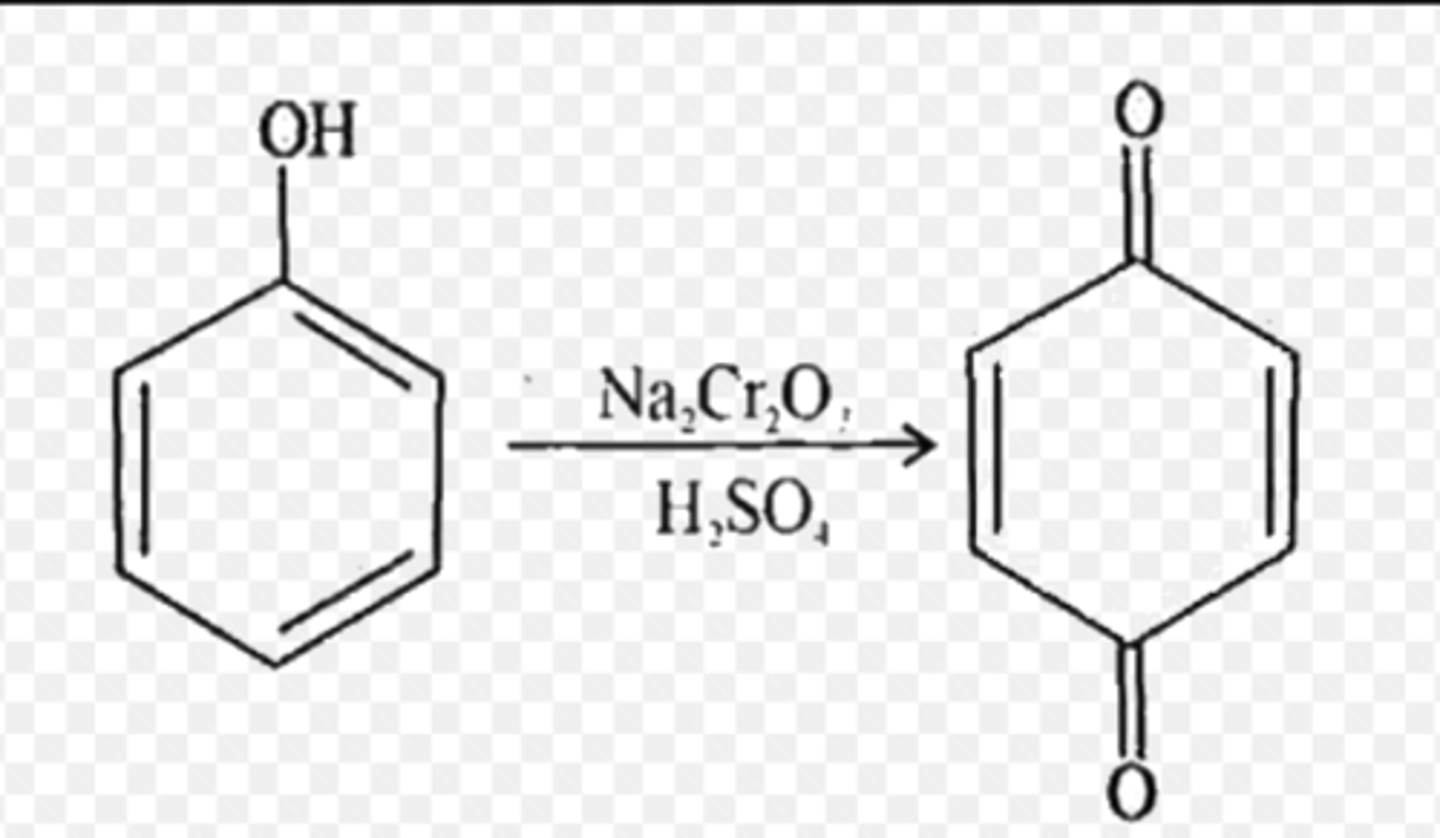
action of chromic acid on phenol
forms benzoquinone
give an example of Fries rearrangement