Lecture 5 - DNA Damage Signalling
1/78
Earn XP
Description and Tags
ONCOL 335 - Radiobiology. University of Alberta
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
79 Terms
Review: what is the DNA damage response?
all of the processes that happen after cell receives DNA damage
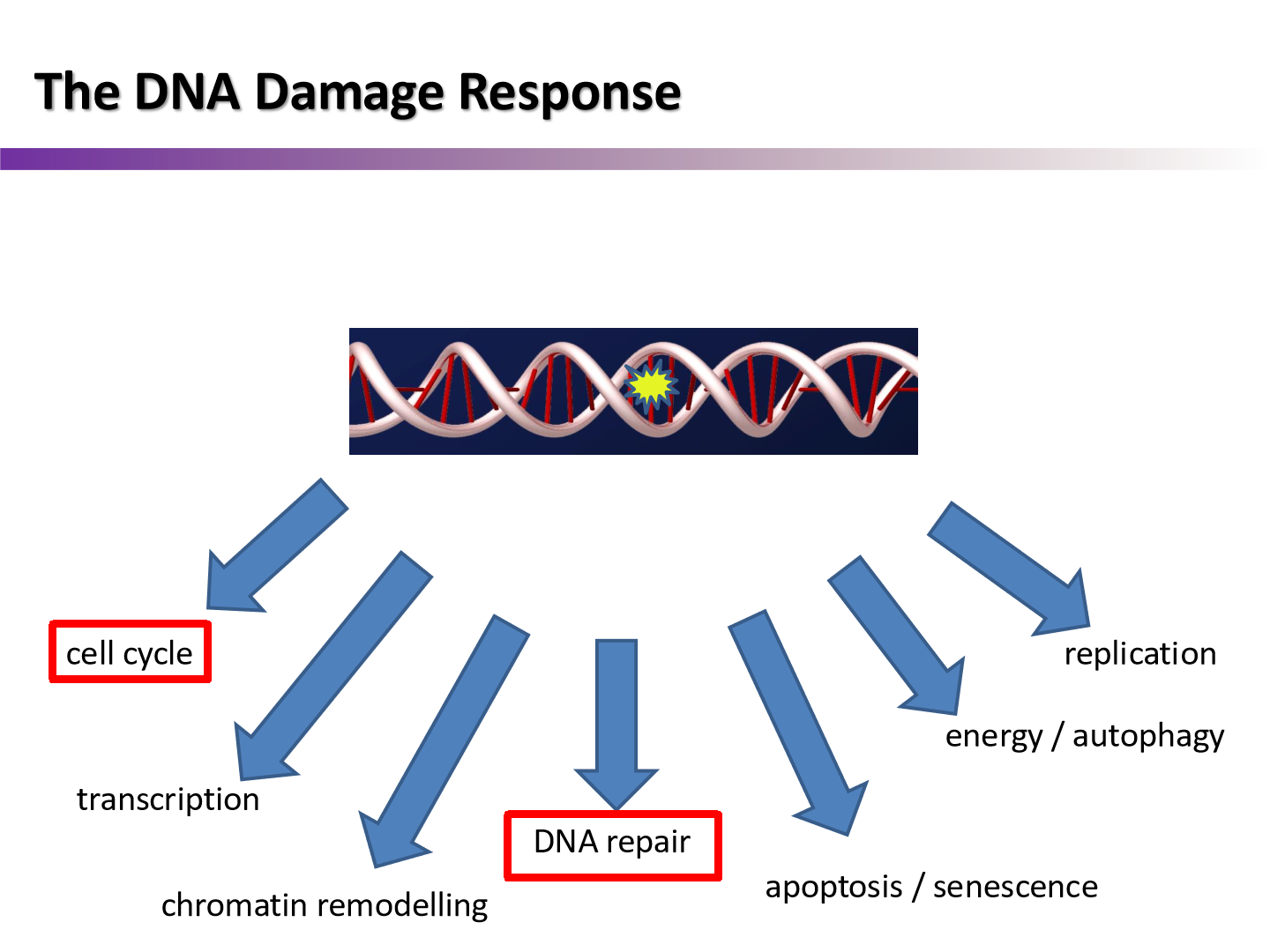
what are the three categories of genes responsible for signal amplification?
Sensors: sense DNA damage
Transducers and Mediators: amplify the signals
Effectors: do the work
Examples of Sensors
NHEJ: KU 70/80
HR: MRN complex
ssDNA present: RPA coats DNA strands
What transducers/mediator does Ku 70/80 activate
DNA-PKcs
What transducers/mediator does MRN complex activate
ATM
What transducers/mediator does RPA activate
ATRip/ATR
What do kinases play an important role in?
signalling cascade
what two kinases are apical (top of signalling cascade)
hint: DNA damage sensors activate them
ATM and ATR
ATM phosphorylates/activates ___
CHK2
ATR phosphorylates/activates ___
CHK1
although phosphorylation and ubiquitination are the only modifications discussed, what other modifications can occur?
methylation and acetylation
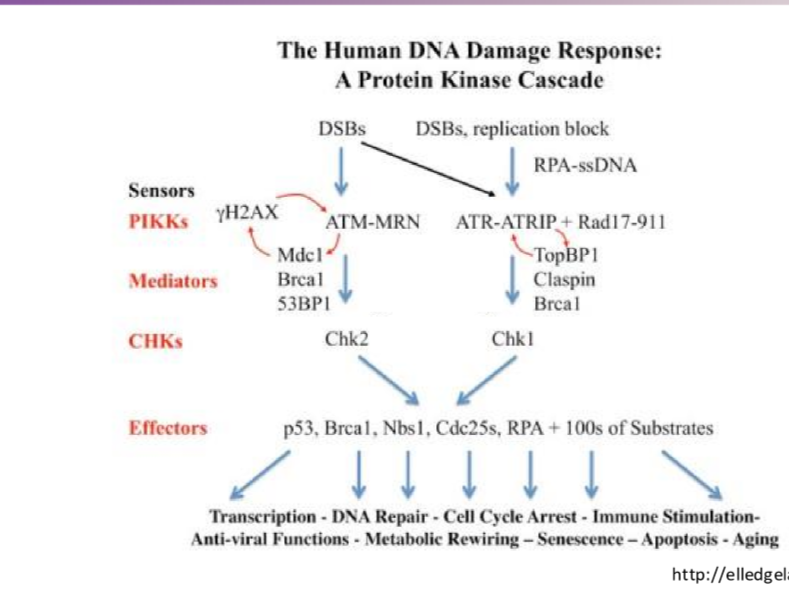
Describe the simplified prtoein kinase cascade after radiation for ATM
DSBs activate ATM —> CHK2 —> p53 (transcription halt), BRCA1 (DNA repair), RPA (cell cycle arrest) + others
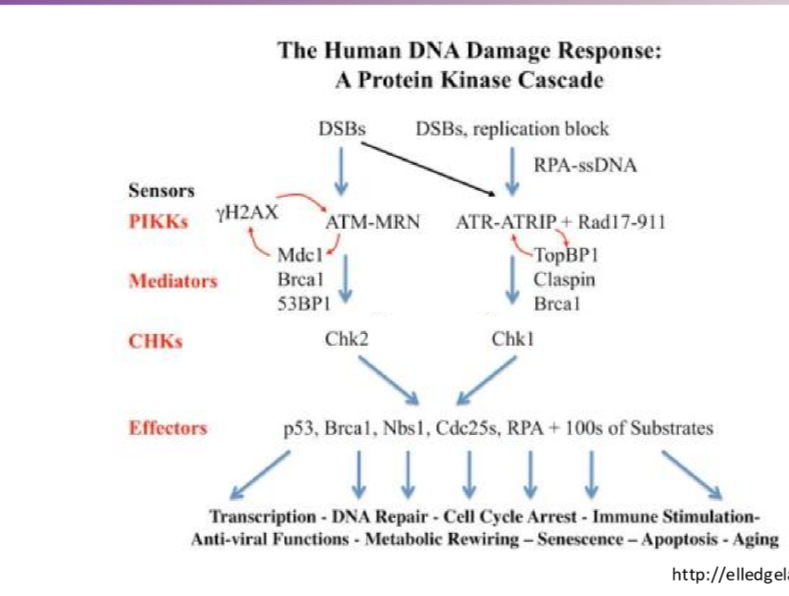
Describe the simplified prtoein kinase cascade after radiation for ATR
DSBs activate ATR —> CHK1 —> p53 (transcription halt), BRCA1 (DNA repair), RPA (cell cycle arrest) + others
Ataxia telangiectasia (AT) is a rare ___ ___ disorder
autosomal recessive
symptoms of AT
neurodegeneration, immunodeficiency, increased risk of lymphoid malignancies (due to DNA damage signalling pathways inhibited) and interstitial lung disease
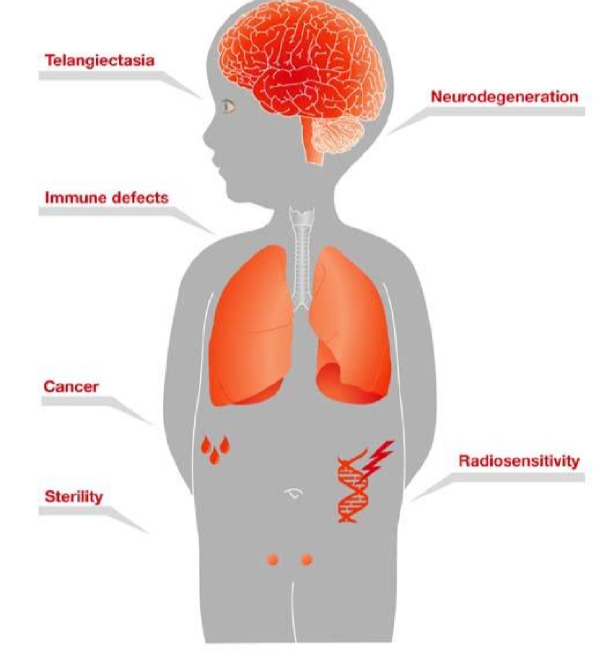
AT effect on radiosensitivity
makes patients extremly radiosensitive, can’t give them radiotherapy if they have cancer
What gene is mutated in AT?
mutations in ATM gene, patients have no ATM protein
Seckel syndrome is a rare ___ ___ disorder
autosomal recessive
symptoms of seckel syndrome
bird like face, postnatal dwarfism, microcephaly, joint malformation, intellectual disability
Seckel syndrome effect on radiosenstivity
moderate, but still make patients radiosensitive
what gene is mutated in seckel syndrome?
ATR gene mutation, causing low levels of ATR
in AT, patients have no ATM protein, but in seckel, they have low levels of ATR. why doesn’t seckel cause complete removal of ATR protein?
ATR is an essential protein
why is ATR an essential protein?
because ATR is recruited after RPA coats ssDNA after UV light replication stress, if ATR is not present, genome would be damaged
thererfore if person had no ATR, they would probably be dead
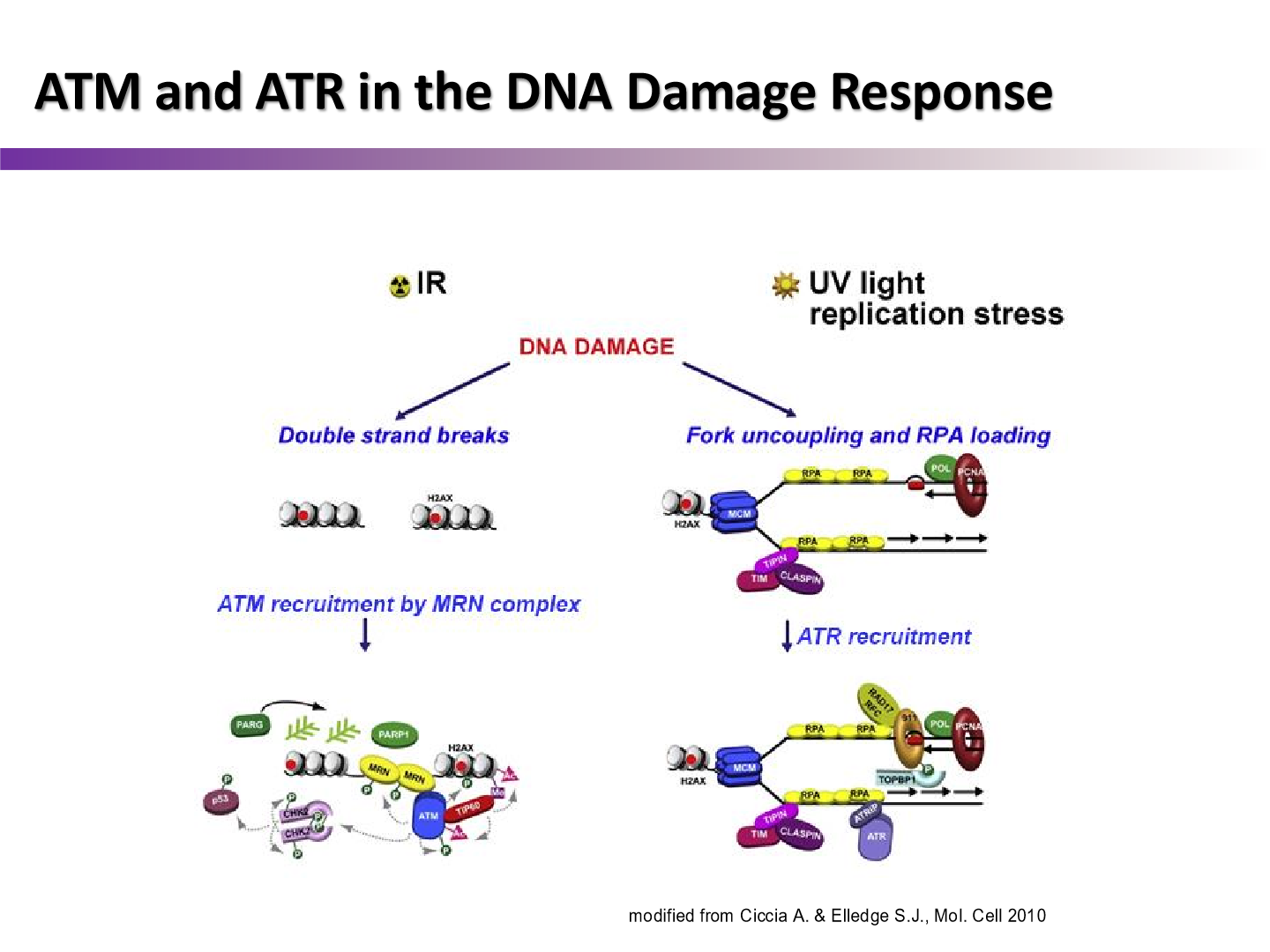
How many substrates do ATM and ATR kinases activate?
between 700 and 1000
how many cell cycle checkpoints do we have in our cells?
4
G1/S
S
G2/M
M
which checkpoint is the most important?
G2/M
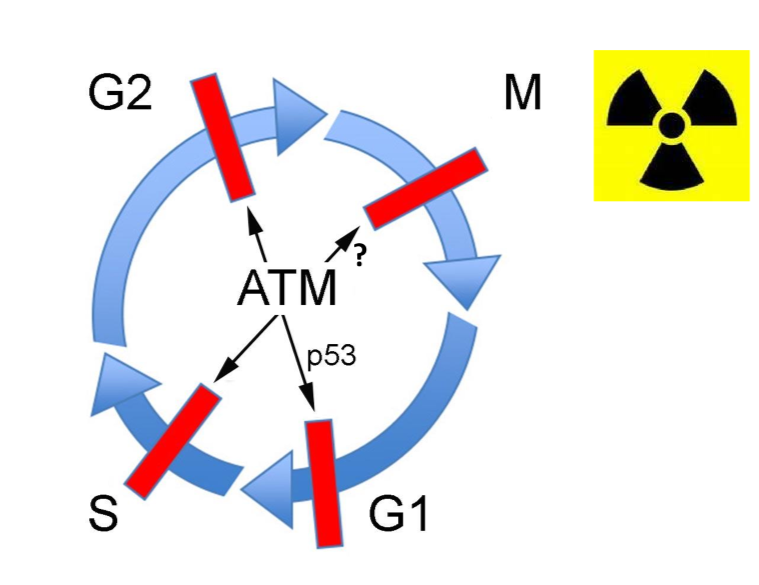
Which checkpoints are regulated by DNA damage, and therefore regulated by ATM?
all of them except M phase
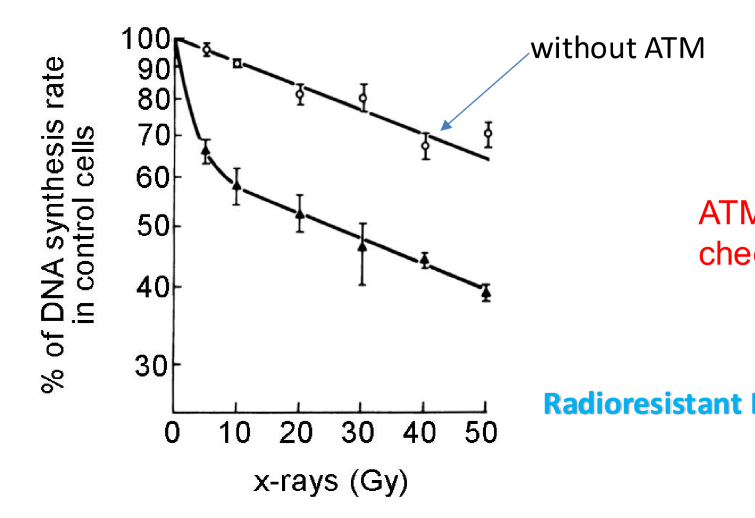
How does DNA synthesis change with ATM mutation?
cells with ATM mutation continued to go through S phase checkpoint while normal cells stopped to repair DNA
therefore ATM is required to inhibit DNA synthesis after radiation exposure
the more radiation dose delivered to the cell, what happens to ATM activation?
more ATM proteins are activated!
how many DSB breaks happen due to 1 Gy of radiation?
40 breaks
how many DSB breaks happen due to 0.05 Gy of radiation?
2 breaks
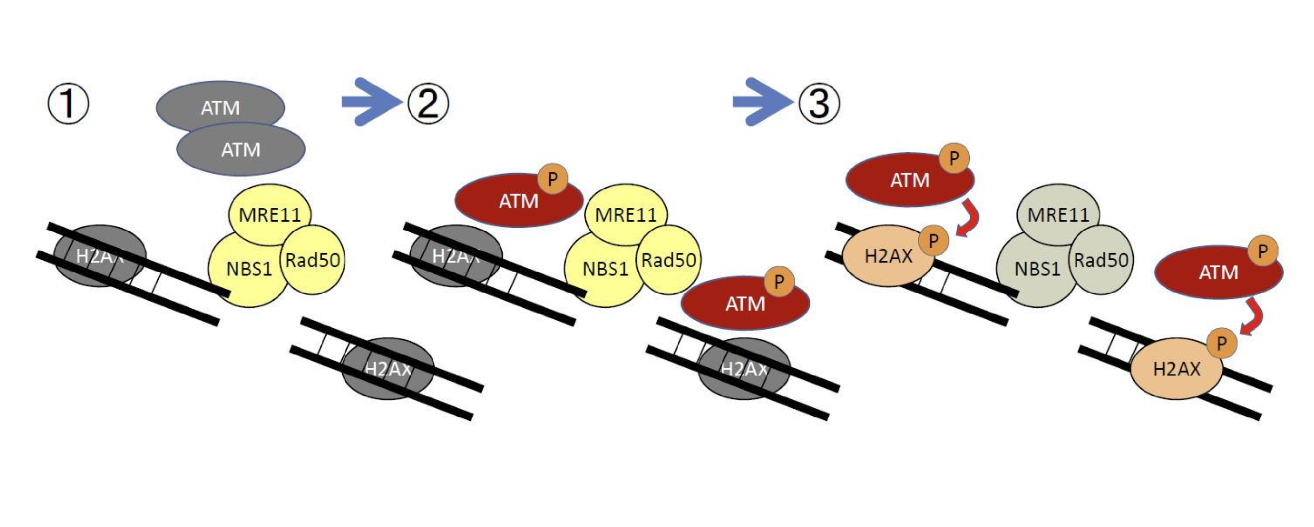
Describe how ATM is activated?
ATM is recruited my MRN complex, and then ATM phosphorylates itself to activate
what is p53?
P53 is a transcription factor: a protein that binds to a certain site of gene and then brings in the machinery to read gene (like helicase and polymerase)
How p53 activated?
ATM/ATR will phosphorylate p53 in addition to CHK2/1 after DSBs
- CHK2/1 will also phosphorylate p53
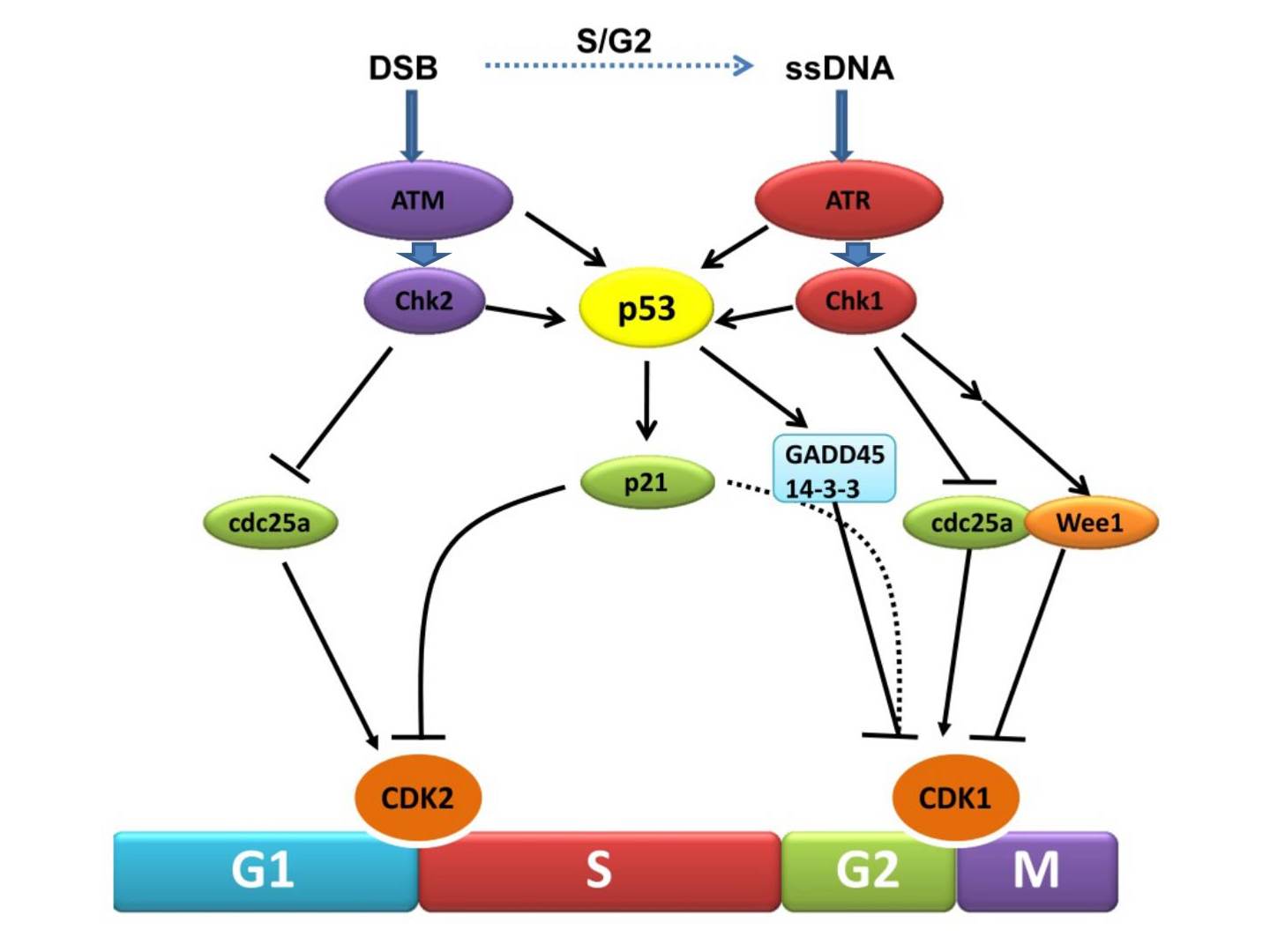
What does p53 activate?
p53 activates p21, and p21 inhibits CDKs: preventing the cell cycle from progressing
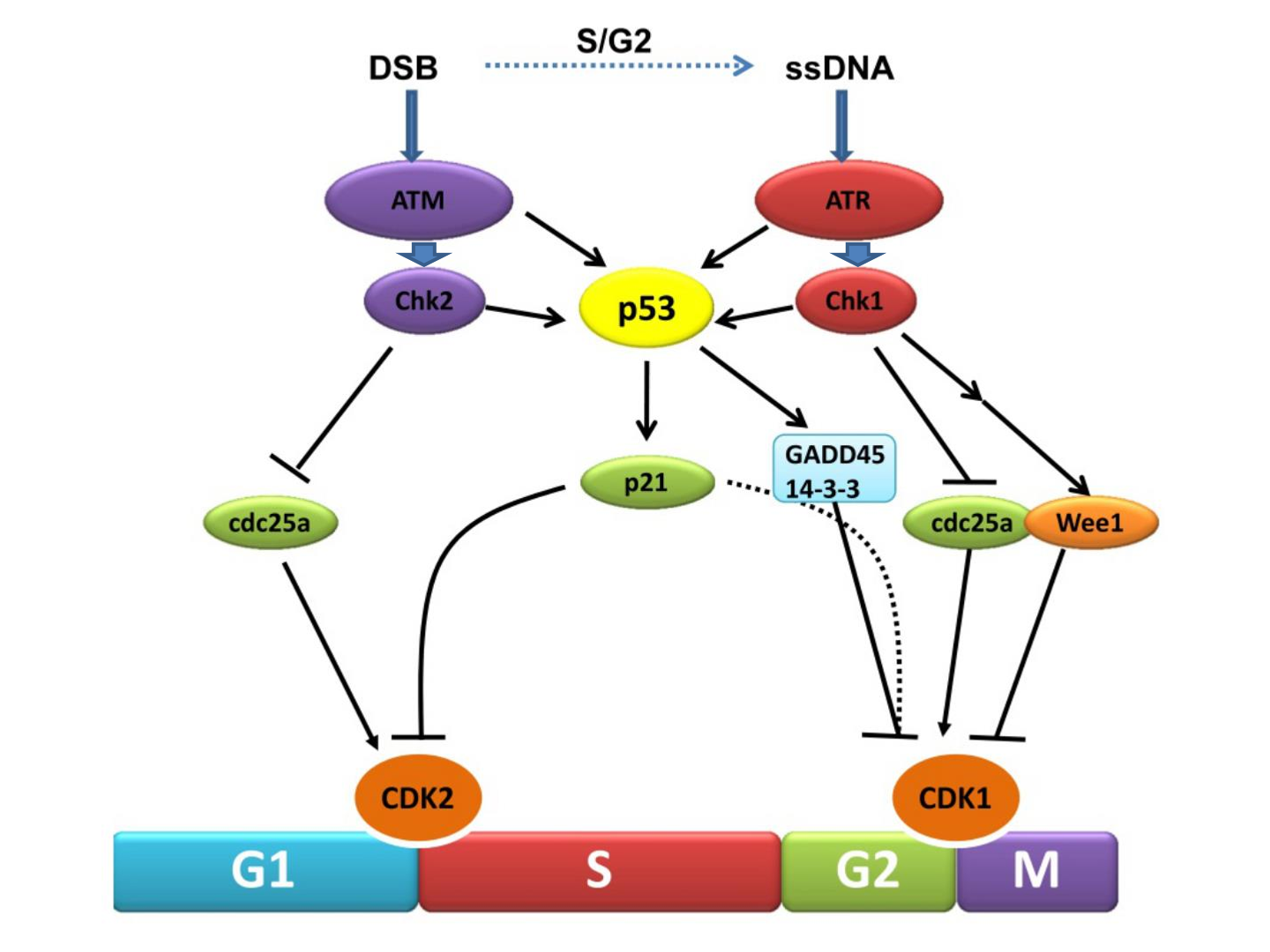
what stops G1 checkpoint
damaged DNA
what stops S phase checkpoint
unreplicated or damaged DNA
What stops G2/M checkpoint
unreplicated or damaged DNA
what stops M checkpoint
chromosome misalignment
primary signalling proteins in each checkpoint
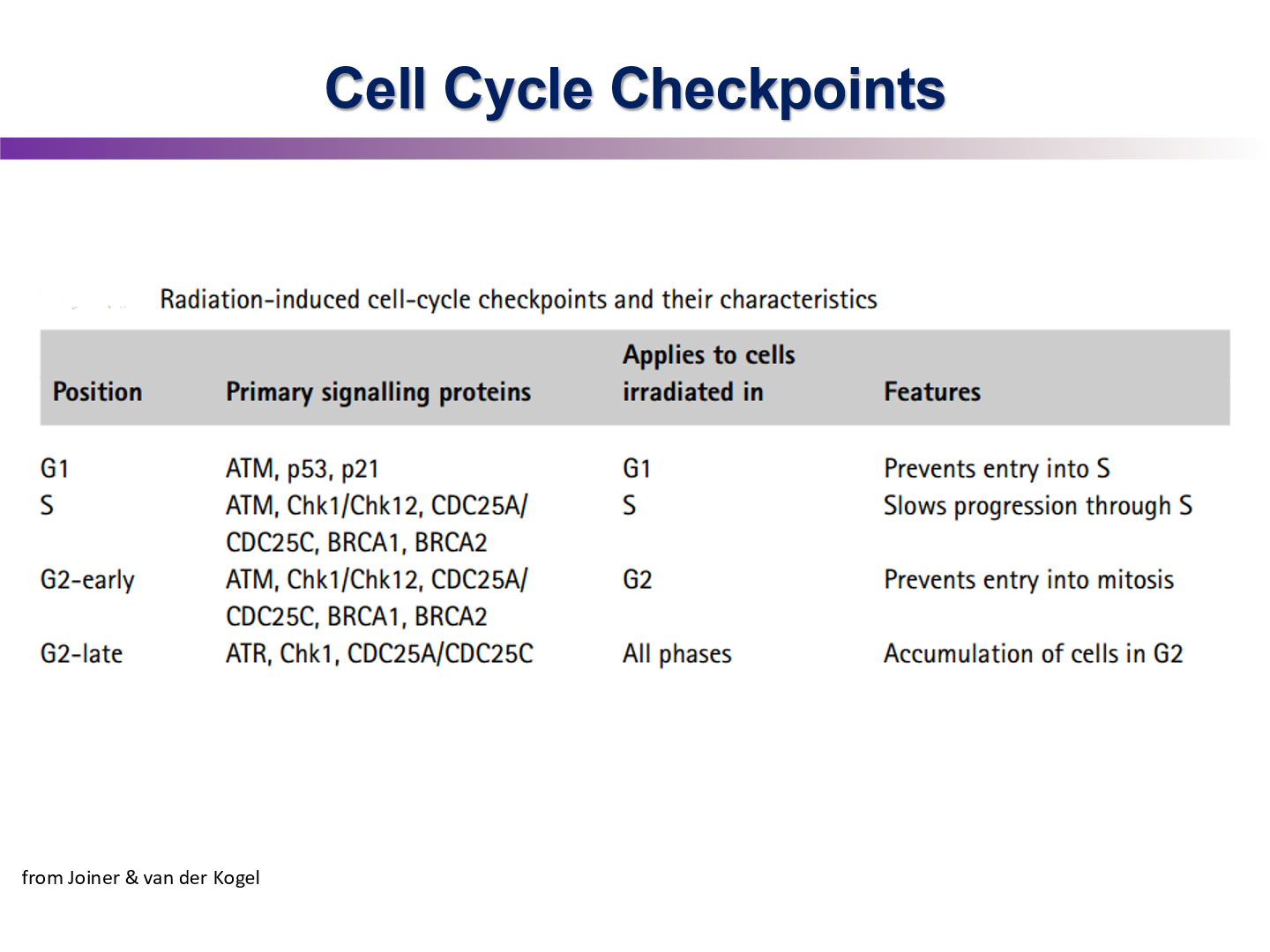
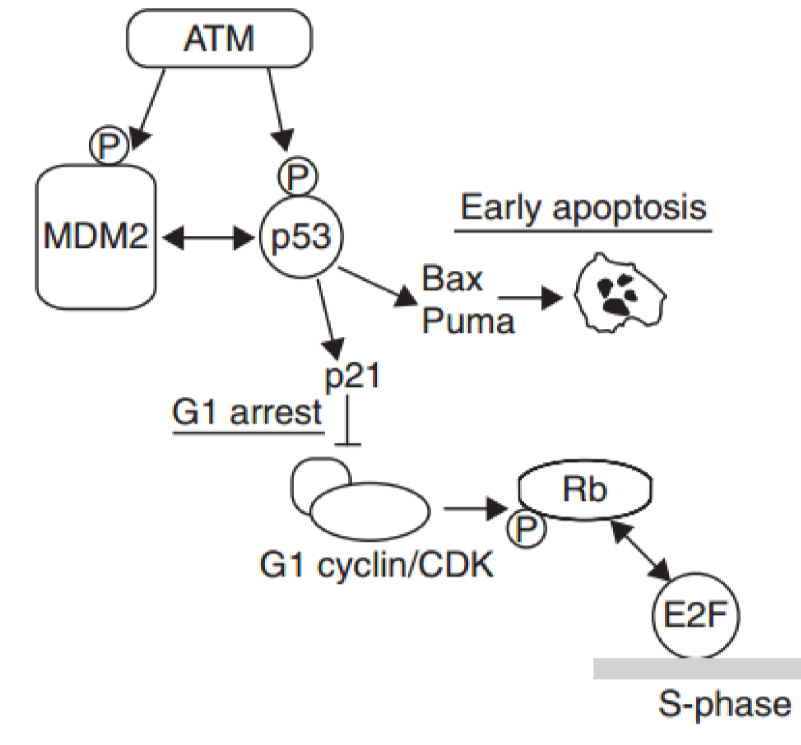
Describe the DNA damage pathway starting with ATM
ATM is recruited by the MRN complex. ATM then phosphorylates CHK2. CHK2 then will activate cdc25 phosphatase by removing phosphate group. Phosphatase will then remove a phosphate from CDK —> leading to cell cycle progression
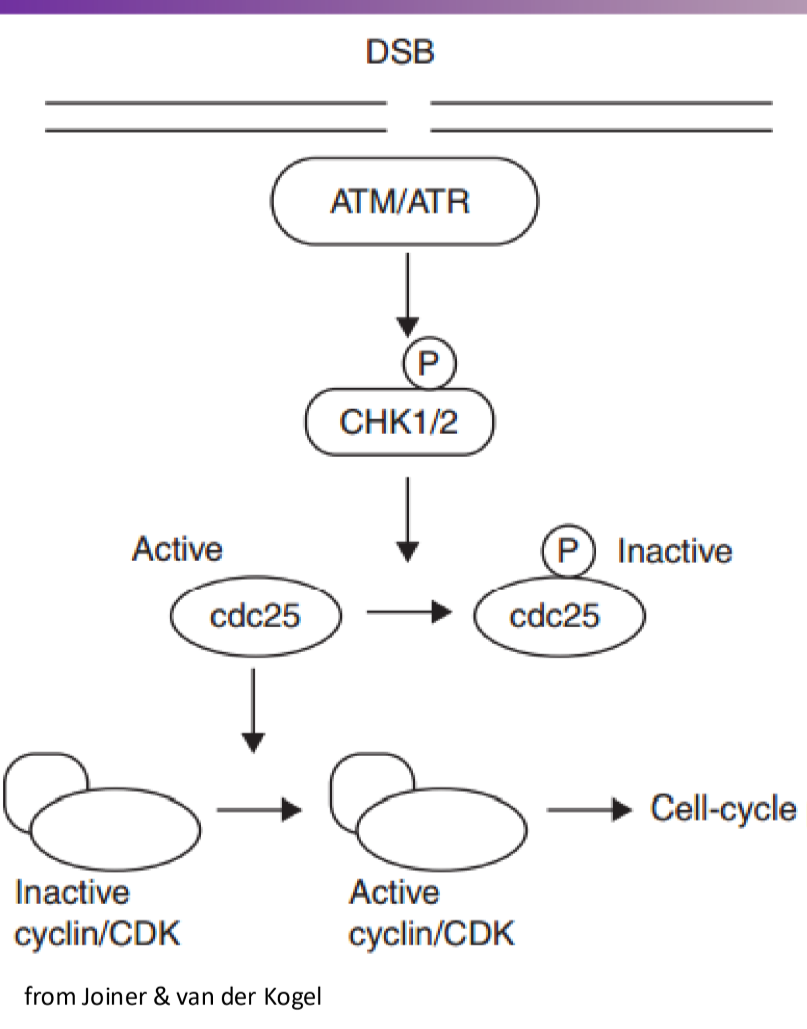
How is the cdc25 phosphatase inactivated?
phosphatases are inactivated by adding a phosphate group to them, and then it will get marked to degradation
What is p53?
a transcription factor that regulates genes involved in cell cycle progression (p21), apoptosis (PUMA), autoregulation (MDM2)
how transcription factors work
they contian 2 domains: a DNA binding domain and a functional domain that recruits transcription equipment to that certain portion of DNA
Describe how p53 is activated
p53 is activated when there are cell cycle abnormalities, DNA damage, and hypoxia. It then will regulate genes in cell cycle arrest, apoptosis, etc
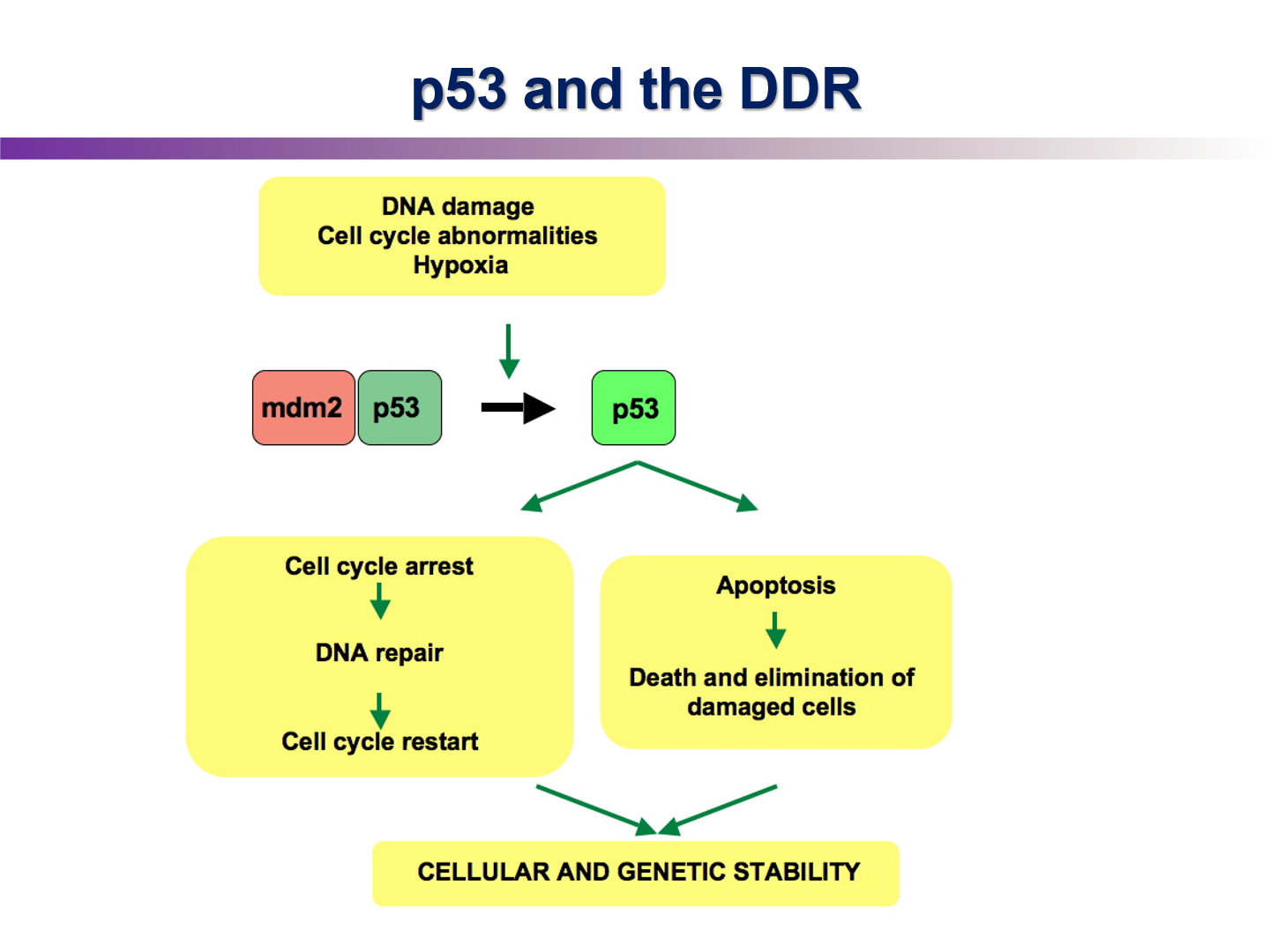
Describe the p53/MDM2 autoregulatory feedback loop
when p53 is activated, it binds to target genes such as p21 and PUMA, but it also binds to and activates MDM2, a ubiquitination ligase
MDM2 adds Uv tags to p53 which is a signal to the cell to degrade it
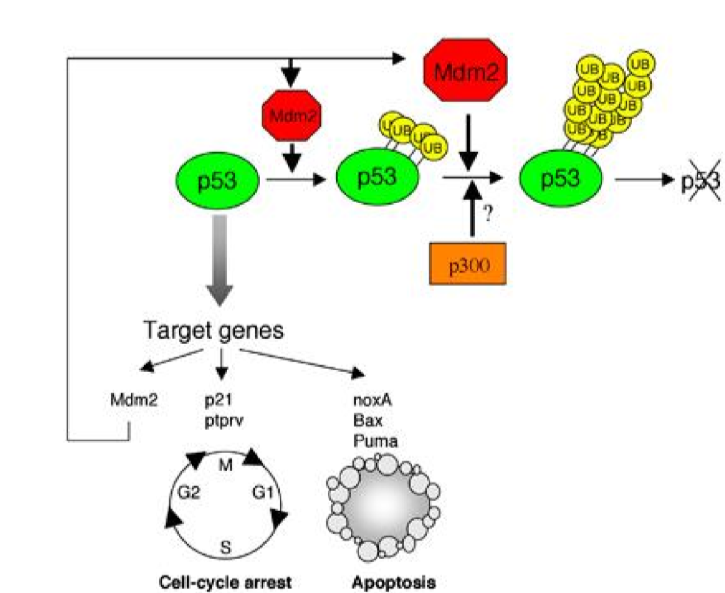
If p53 activity increases, what happens to MDM2 activity?
it will also increase to get rid of p53 after the cell cycle can begin again
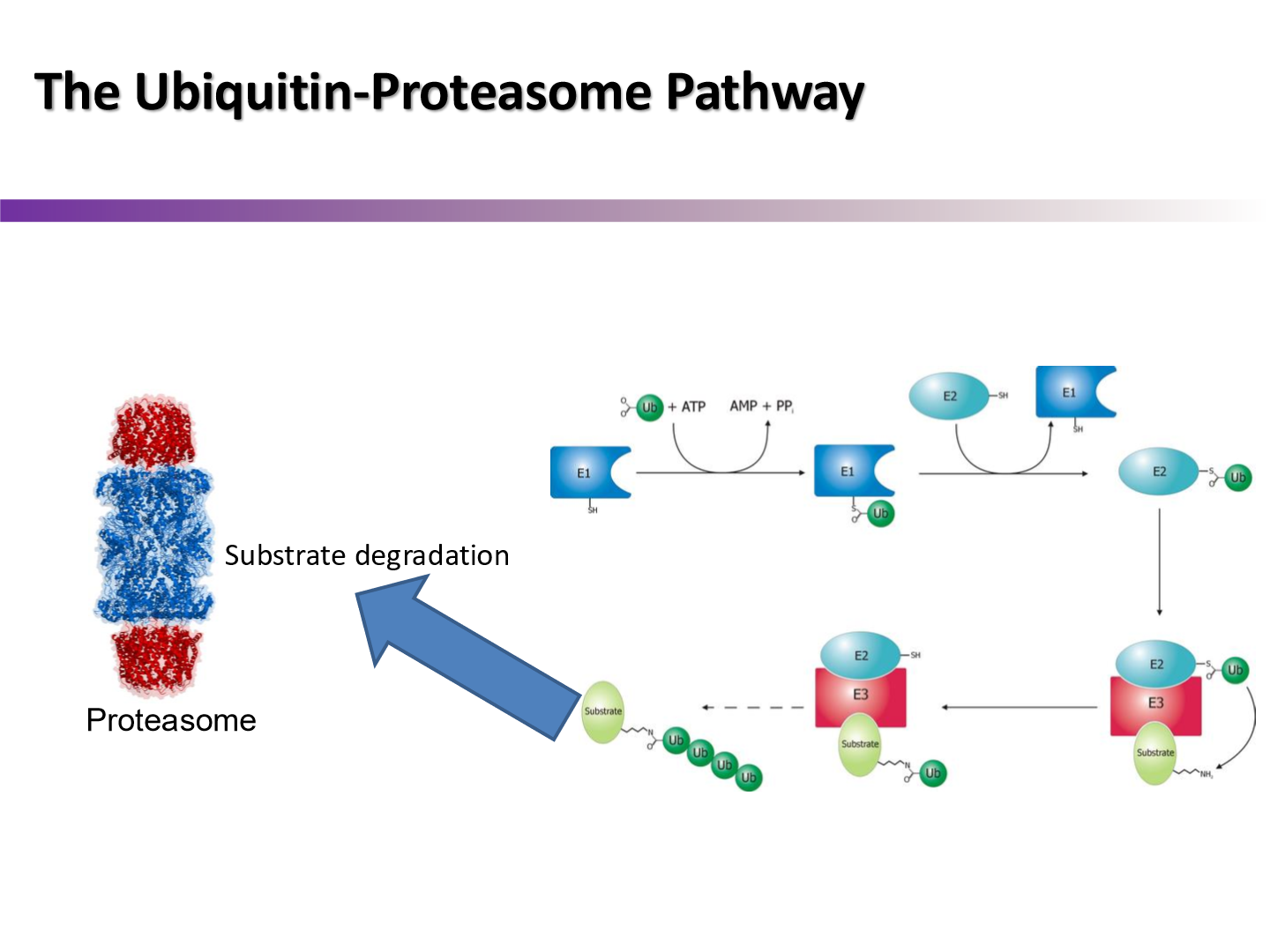
Describe the ubiquitination-proteosome pathway MDM2 uses to ubiquitinate p53
when the E3 domain of the MDM2 ligase recognizes p53, it binds to it. the E2 domain of MDM2 then adds the Ub tail to p53, resutling in p53 degradation at the proteosome
Why does ATM phosphorylate MDM2 after DSB are detected?
Mdm2 is phosphorylated by ATM as a safe guard so p53 cannot be targeted until p53 activates it
P53 activates p21, what role does p21 play in G1 cell cycle arrest
p21 is a CDK inhibitor
in a normal cell cycle, when G1 cyclin/CDK is activated by cdc25 phosphatase, it will phosphorylate the Rb protein
as a result Rb protein lets go of the E2F transcription factor, so S-phase genes are created
without E2F cell can’t enter S phse, so p21 ensures E2F is not activated
what is the most common tumor supressor mutation in cancer?
p53 mutations
What cancers have the highest mutation rates
Micro Satellite Instability Colorrectal, melanoma, and lung cancer
what cancers have the lowest mutation rates
pediatric cancers passed down through germ cells
ex: glioblastoma
how many cell signalling pathways are affected in cancer?
there are 12 primary pathways affected by cancer that can be split into three categories
Cell Survival
Genome Maintenance
Cell Fate
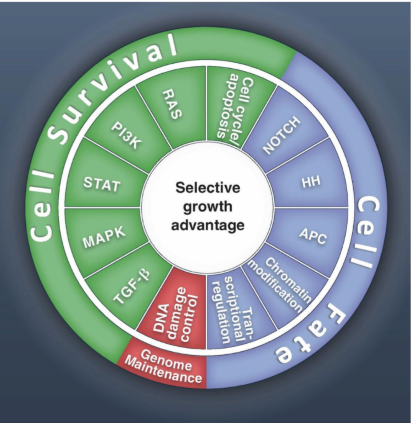
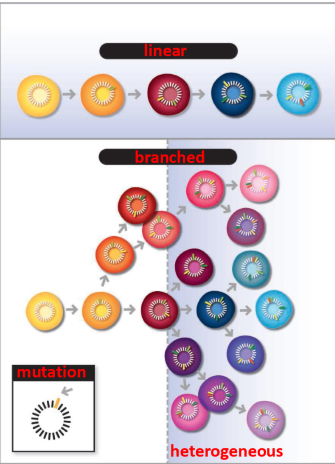
is tumor evolution linear or heterogenous
it is heterogenous
All clones may be slightly different, changing response to drugs and radiation
how can the p53 be inactivated/mutated
loss/mutation
mutations in DNA binding or gain of function
MDM2 overexpression
viral oncogenes: proteins that bind to p53
what cancer has least association with p53
cervical cancer
as it is associated with HPV
Describe the pathway that HPV effects p53
HPV viral protein E6 binds to p53 and inactivates it
Somatic Mutation - definition
a change in the DNA sequence of a somatic cell of a multicellular organism with dedicated reproductive cells
therefore not passed on to children
sporadic cancer - definition
Cancer that occurs in people who do not have an inherited genetic variant that would increase their risk for that cancer.
‘random mutations’
Are most cancers hereditary or non-heriditary
non hereditary (sporadic)
Ataxia telangiectasia
a rare autosomal recessive disorder that leads to neurodegeneration, immunodeficiency, sterility and increases radiosensitivity of tissues
what gene is mutated in AT
both ATM genes must be mutated
Nimegan Breakage Syndrome
a autosomal recessive disorder characterized by progressive microcephaly, early growth deficiency that improves with age, recurrent respiratory infections, an increased risk for malignancy
what gene is mutated in Nimegan Breakage Syndrome
NBS1
Severe Combined Immunodeficiency (SCID)
a genetic disorder characterized by disturbed development of T and B cells
patients susceptible to diseases, viruses, etc
need to live in bubble and receive bone marrow transplant
what genes are mutated in SCID?
mutations in NHEJ genes
Artemis
Ligase IV
XLF
Fanconi Anemia
an autosomal recessive disorder characterized by developmental abnormalities, bone marrow failure and AML
also have issues with hands: a shortened or absent thumb, radius, or both
Defining feature of fanconi anemia
early bone marrow failure due to interstrand cross links
What gene is mutated in Fanconi Anemia?
genes in the FA family: FANCD1 and FANCS
inter-strand crosslinks of DNA cannot be repaired
Li Fraumeni Syndrome
rare, autosomal dominant, hereditary disorder that predisposes carriers to sarcoma, breast, leukemia, and adrenal gland cancers
Li Fraumeni Gene mutations
p53 or CHK2
what reccomendation should be made to patients with Li-Fraumeni in regards to radiation therapy
avoid radiation therapy to reduce risk of secondary radiation induces tumors
Define heterozygous in terms of genetics
Aa: person has two different alleles of same gene
Are ATM mutation carriers homo or heterozygous
heterozygous
if person had both sets of mutated ATM, they would be dead as they would have ataxia telangietasia
Are ATM heterozygous people (Aa) more susceptive to radiation than AA people?
results are contradictory in literature, we are not sure
Are ATM heterozygous people (Aa) more susceptive to breast cancer than AA people?
Yes, they are at a little higher risk than AA people
risk ratio = 3.3
What is the risk ratio
example
RR = (pbty of getting cancer when you smoke)/(pbty of getting cancer when you don’t smoke)
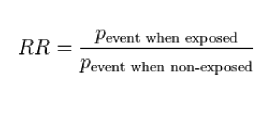
what is the RR for getting breast cancer if you have a BRCA mutation
200: 200 times more likely to get breast cancer if you have BRCA mutation