carbonyls
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
28 Terms
What defines carbonyl compounds?
Presence of C=O bond
Describe carbonyl structure
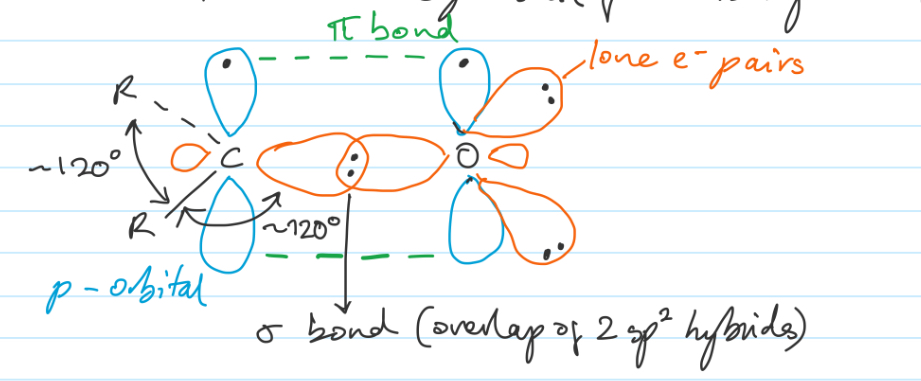
Trigonal planar sp2 hybridised C and O
All sigma bonds lie in same plane about 120° apart
C-O pi bond between parallel p orbitals
Which is the bond responsible for carbonyl reactivity?
Pi bond between parallel C and O p orbitals
Draw the basic structure of a carbonyl group
Compare the C=O bond to C=C in alkenes
C=O is shorter, stronger and more polar
in what ways could a carbonyl compound interact with binding sites in the body?
Hydrogen bonding - C=O is HBA (O can accept 2 h bonds)
Dipole-dipole interactions due to dipole moment
Describe in general how reactions occur at the carbonyl carbon
The electron pair moves from C=O to more electronegative O
After nucleophilic attack
Produces a tetrahedral alkoxide ion intermediate
Formation of new bonds increases steric crowding
how and why does a nucleophile approach carbonyl carbon?
From either above or below plane of C=O
C is slightly positive and electrophilic due to polar C=O bond
This attracts nucleophiles
Are aldehydes or ketones more reactive, and why?
Aldehydes more reactive
Less steric crowding so Nu- only has to pass by 1 side chain (vs 2)
How can reactivity of carbonyls be enhanced?
By protraction of carbonyl O to make the conjugate acid
Proton attached to O draws electrons over O making C more positive
The C becomes more electrophilic and is now more susceptible to nucleophilic attack
Define a hydrogen bond
Short range, directional, inter or intramolecular non-bonded interaction
Between H and lone pair on one of N, O or F
Outline how hydrogen bonding occurs between carbonyl compounds and water
Between 2 lone pairs on delta negative O and delta positive of water
H is h bond donor, O on C=O is acceptor
Outline the importance of hydrogen bonding of carbonyl compounds as drugs
Receptor interactions within the body
Cell components largely made up of hydrogen bonds eg, proteins, water
Carbonyls on drug molecules are able to disrupt this and have an effect
Which form of nucleophilic addition is easier to reverse?
Under acidic conditions
Describe what happens during the reversal of nucleophilic addition under basic conditions
Base (OH-) removes proton on -OH group
O- reforms C=O
Carbonyl formation with loss of leaving group (Nu)
How do carboxylic acids behave in water?
Weak acids - proton donors
Transfer a proton to water to give H3O+ and carboxylate anions R-COO-
Describe what happens during the reversal of nucleophilic addition under acidic conditions
Protonation of original Nu species
Loss of leaving group (Nu-H)
-OH group becomes C=O as loses proton
Give the pKa range for aliphatic and aromatic carboxylic acids
3-5
Why do carboxylic acids have greater acidity than alcohols?
Due to resonance stabilisation of the COO- anion

If a drug has a low pKa at what pH and where would its ionisation be greatest?
At alkaline pH
Small intestine
If a group has an electron-donating inductive effect, will this stabilise or destabilise the anion it’s attached to?
Destabilise
Name a reducing agent you could use to reduce aldehydes or ketones
LiAlH4
Describe how aldehydes and ketones react to oxidation
Aldehydes - to carboxylic acids with oxidising agent eg, CrO3 or KMnO4
Ketones - inert towards oxidation
Why will compounds with pKa higher than water (>16) not ionise in water?
As the water will be the stronger acid
H3O+ is stronger so reverse reaction (proton transfer back to the compound trying to ionise) is more stable
Thermodynamically favourable to form the weaker acid
Why are acyl chlorides the most reactive carbonyl derivative?
Hybridisation during resonance - better overlap of 2p orbitals of C and O compared to 3p and 2p of Cl and C
Cl- is a better leaving group (than -O-R) as it is the weak base of its strong conjugate acid HCl, and is large and electronegative enough
Explain the difference in acidity between fluoroacetic acid (CF3COOH) and acetic acid (CH3COOH)
Fluoro stronger acid (lower pKa)
Electronegativity on F pulls electron density towards CF3 making it easier to lose H+ on -OH group
CH3 is slightly electron releasing so more difficult to lose H+
State the steps of acid catalysed hydrolysis of esters
H+ added to C=O, lone pair nucleophile on water attacks electrophilic carbon, C=O broken (to form C-O-H), e- on O-CH3 attracted to H on nucleophile group, C=O reformed
Alcohol reforms
Lone pair on -OH group reforms C=O bond
Why does nucleophilic substitution not occur when Y group next to C=O is -H or -R (alkyl/aryl group)?
They cannot behave as leaving groups