BIS2A SS1 Week 2
1/111
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
112 Terms
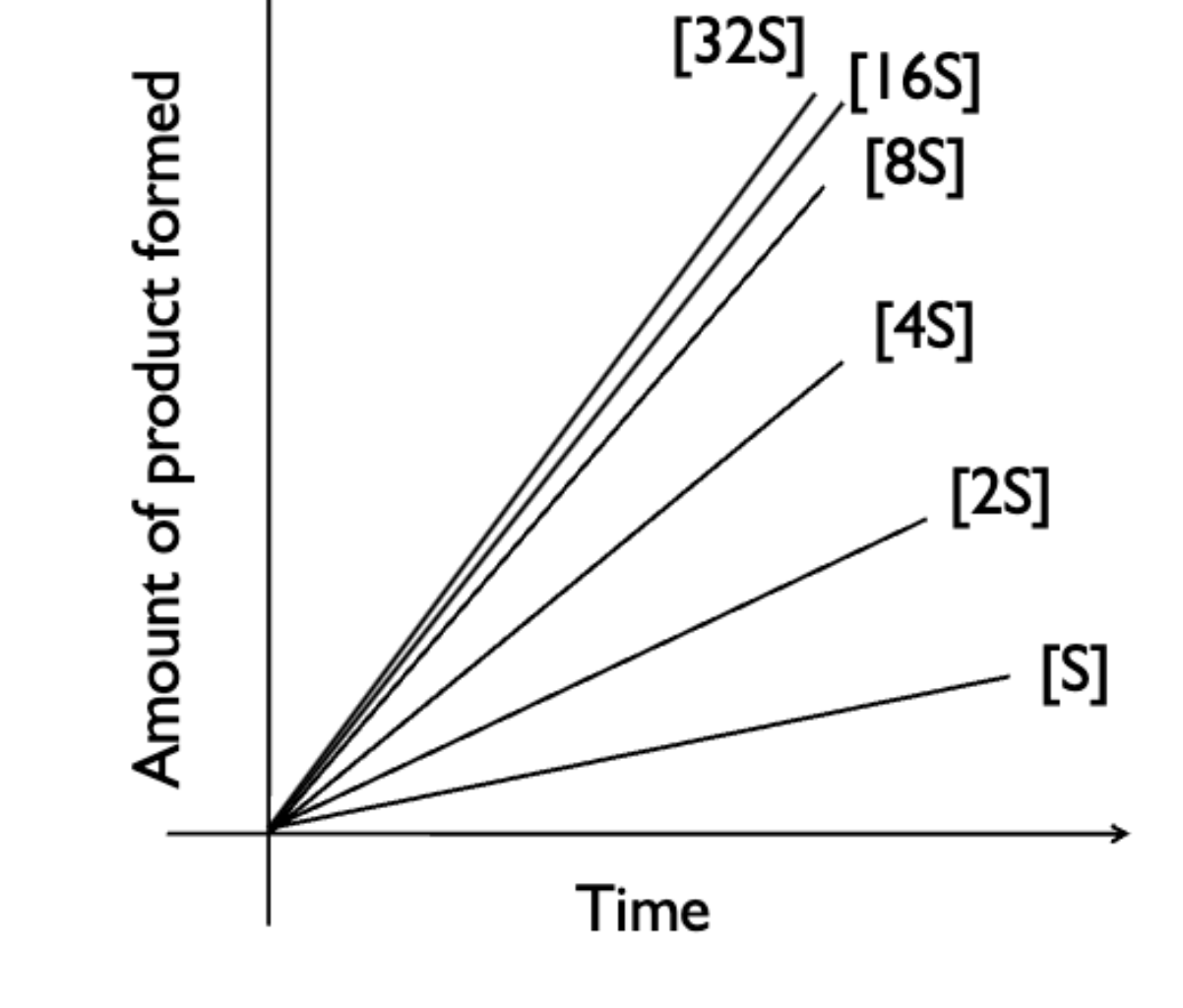
what happen to product formation as you increase the initial amount of substrate and hold enzyme concentrations constant?
when holding enzyme concentrations constant, initially you see a large increase in product formations when going from S —> 2S —> 4S and slows down as enzymes become saturated (increasing initial concentration has less effect)
enzymes — velocity
rate at which product formed (aka how fast the enzyme can catalyze reaction)
Vo
initial velocity when mostly substrate
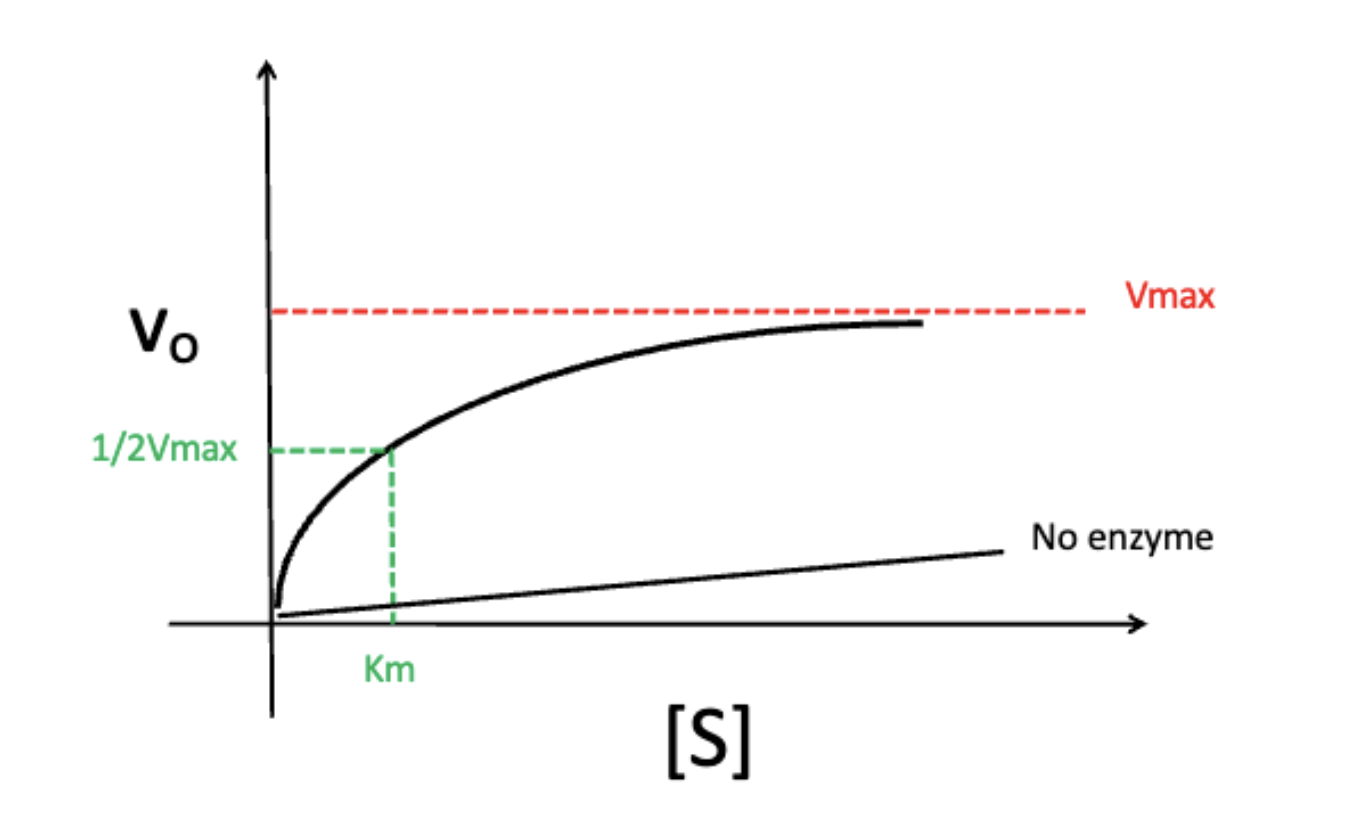
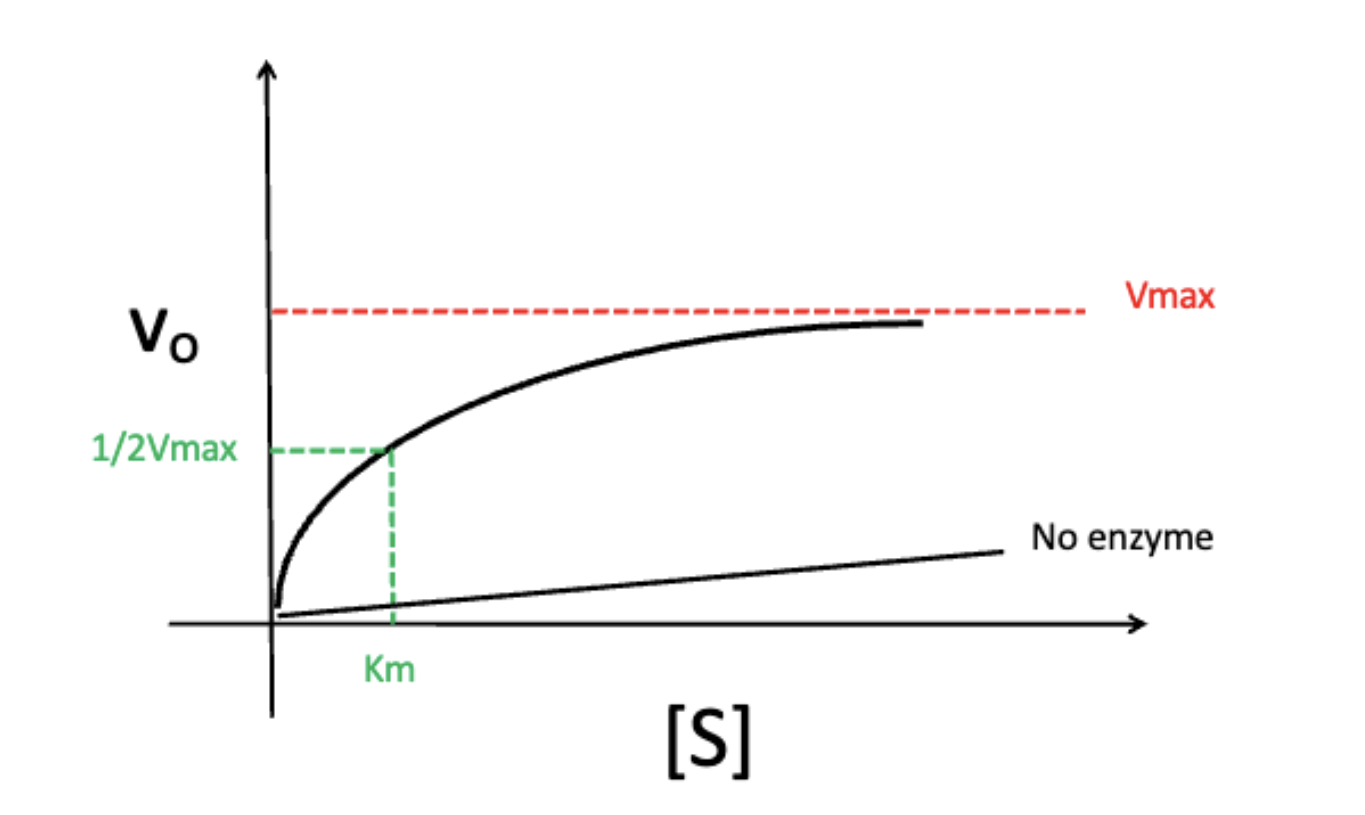
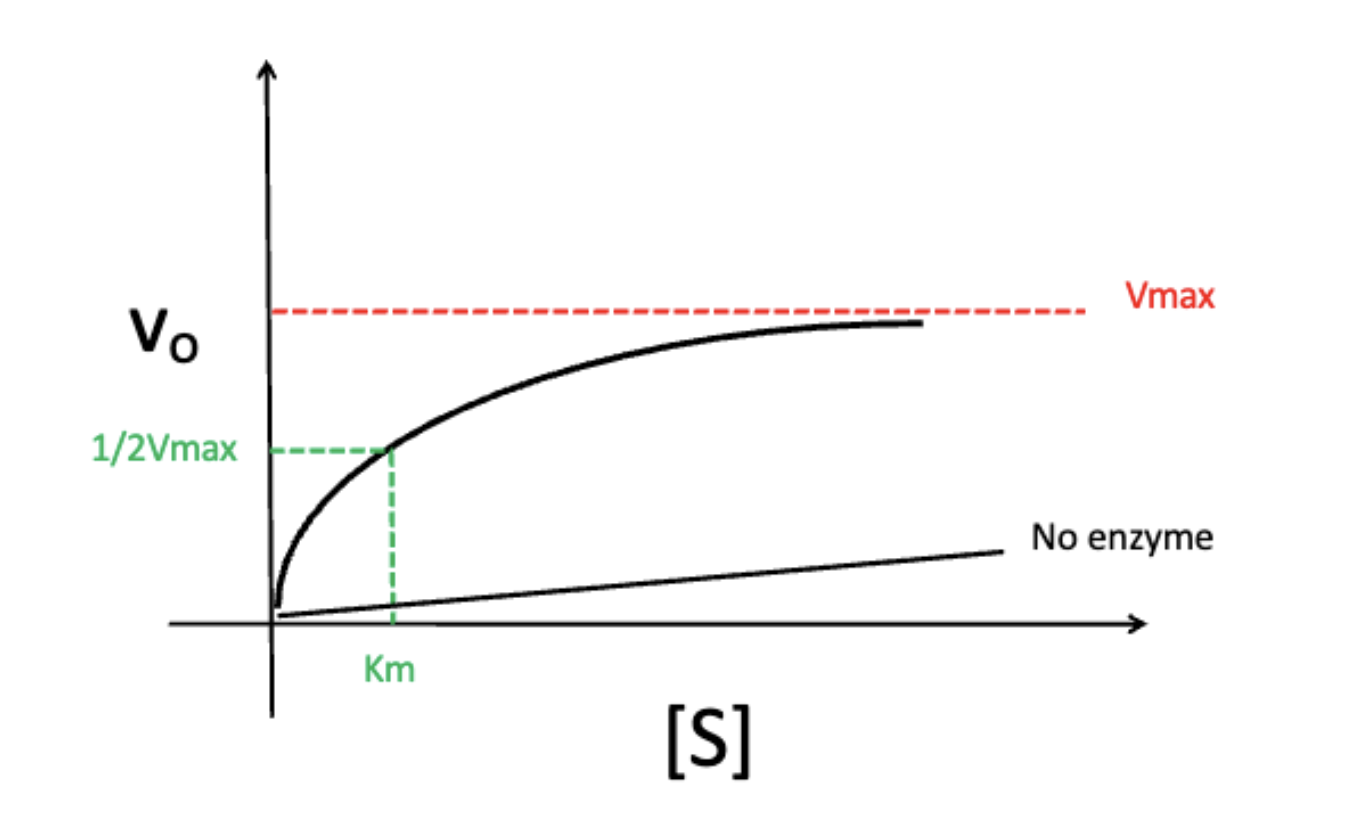
effect of increasing substrate concentration on velocity (Vo)
Vo does not infinitely increase as substrate concentration increases (reaches a maximum aka Vmax)
Vmax
the maximum velocity of an enzyme at which point substrate concentration and rate are independent of each other
Km
at ½ of Vmax
the point at which the enzyme is at the most regulatable state (small changes in substrate concentration affects Vo)
effect of temperature on enzymes
really high temperature —> results in denaturation of protein so rate decreases fast
really low temperature —> rate is slow because reactants don’t collide with enzyme as much
there is an optimal temperature at which an enzyme operates
effect of pH on enzymes
active sites that contain many hydrogen and ionic bonds (acid-base chemistry) will be affected by pH
active site without these chemistries are not affected by pH
allosteric inhibition
activity is regulated (in this case, decreased) by a compound binding to another site (and sometimes the active site)
locks into place the inactive conformation (can be reversible)
allosteric activator
activity increased (active conformation) by binding of a compound to another site (allosteric site) or sometimes the active site
competitive inhibition
blocks activity by binding to the active site and competing with the substrate
functions of the membrane
differentiate between the inside and outside of the cell
semipermeability (regulating what goes inside; more impermeable)
cell-cell recognition
signaling
components of the membrane
phospholipids, integral proteins, peripheral proteins, protein channels, glycoproteins, glycolipids, cholestrol
phospholipids
ester linked glycerol to two fatty acid chains with a phosphate group attached
polar head (glycerol, phosphate group, polar group), 2 nonpolar tails
freely moving/fluid
glycoproteins and glycolipids functions
adhesion, cell-cell recognition
peripheral membrane proteins functions
signaling and cell-cell recognition
integral proteins functions
pumps, channels, carriers (hydrophobic components on outside, hydrophilic inside)
cholestrol function
maintain fluidity at low temps, prevent cell membrane from falling apart at high temps
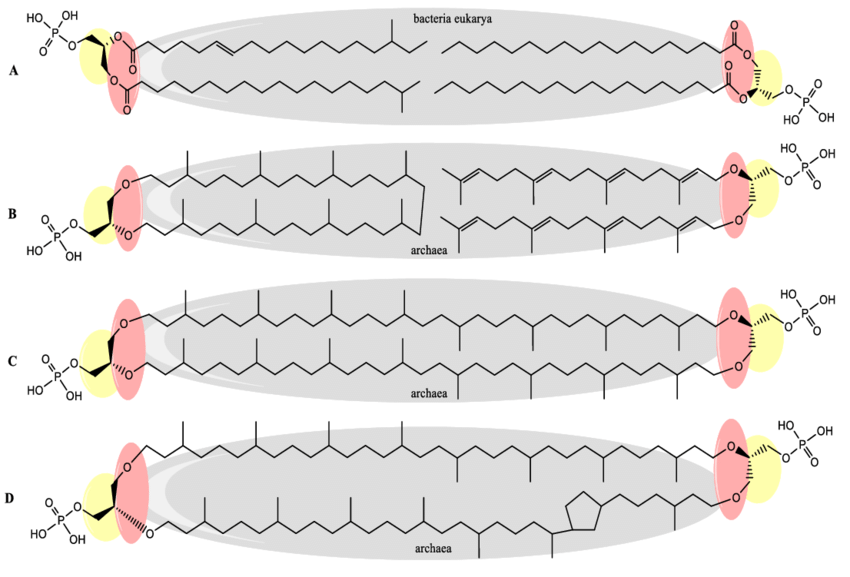
archael phospholipids
ether linked methylated isoprenoid chains (2) to glycerol + phosphate group, can be bilayer or monolayer
permeability of the phospholipid bilayer
small polar molecules can pass through easily (protonated hexanoic acids)
polar/charged molecules cannot pass through/have a harder time passing through
how can you prevent a molecule from inside the cell from passing through the phospholipid bilayer? what is an example of this?
attached a charged group on it, so it cannot pass through the nonpolar/hydrophobic interior of the membrane
ex. attach phosphate group to glucose to prevent it from leaving + creates a concentration gradient so glucose flows into molecule
uniporter
moves one compound in one direction
symporter
moves two molecules in the same direction
antiporter
moves two compounds in different direction (in and out)
passive transport
transport down the concentration gradient — usually until equilibrium is reached
simple diffusion
diffusing through the membrane without the use of anything
few molecules can do this
facilitated diffusion
diffusion down the concentration gradient that requires a channel (doesn’t use energy)
channel protein
restrictive channel that allows certain molecules to go in (they are specific)
used in facilitated diffusion (based on size, shape, charge, etc.)
interior often has polar/charged R groups with properties that facilitate transport; outside is mostly hydrophobic
carrier protein
channel binds to substrate — high specificity
used in both passive and active transport
active transport
transport that requires the use of ATP (primary active) /electrochemical gradient (secondary active) to move substances across the membranes
uses pumps to move against the concentration gradient
active/energized membrane
all cells need an active/energized membrane that has a charge difference
positive charge on outside and negative charge on inside
charge buildup causes protons and another compound to move inside (more negative)
redox pumps
active transport — protons can bring in compounds alongside it as it moves down the electrochemical gradients
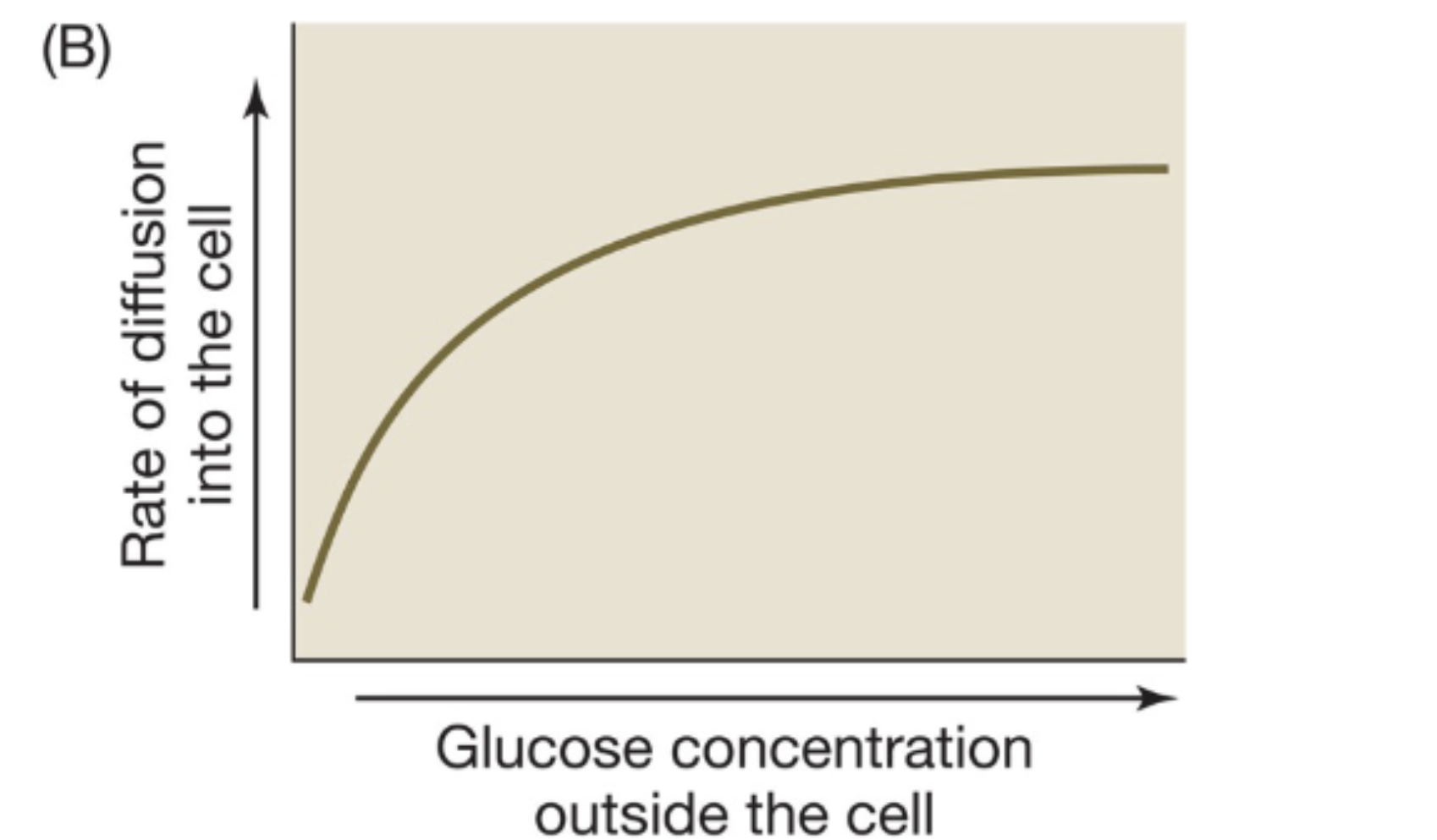
external concentration and the rate of diffusion
greater external concentration increases the rate of diffusion to a certain extent (like an enzyme)
what fibers make up the cytoskeleton?
microfilaments (actin) — smallest
intermediate filaments
microtubules (tubulin) — largest
function of cytoskeleton
cell shape
internal organization
force generation (transporting stuff inside, movement of cell, etc.)
cytoskeleton must be strong/rigid and dynamic
microfilament characteristics
is directional — has a + and - end (not charged; just the direction)
can either go + to - or - to +
helical
made of actin
found on the periphery to give strength, structure, and movement
monomer incorporation and ATP hydrolysis are closely related
microfilament functions
cell shape
movement of entire cell/parts of cell (like in pseudopods/amoeba)
intermediate filaments characteristics and functions
not directional
actin and myosin can create muscle movements
used for almost exclusively structure (typically permanent)
microtubules characterstics
made of tubulin
directional (+ and - end)
growth and shortening of the tubulin depends on GTP hydrolysis
largest + start in center and move outward
microtubules functions
rigid internal skeleton for some cells
framework for movement of motor proteins so cargo can be moved
DNA segregation
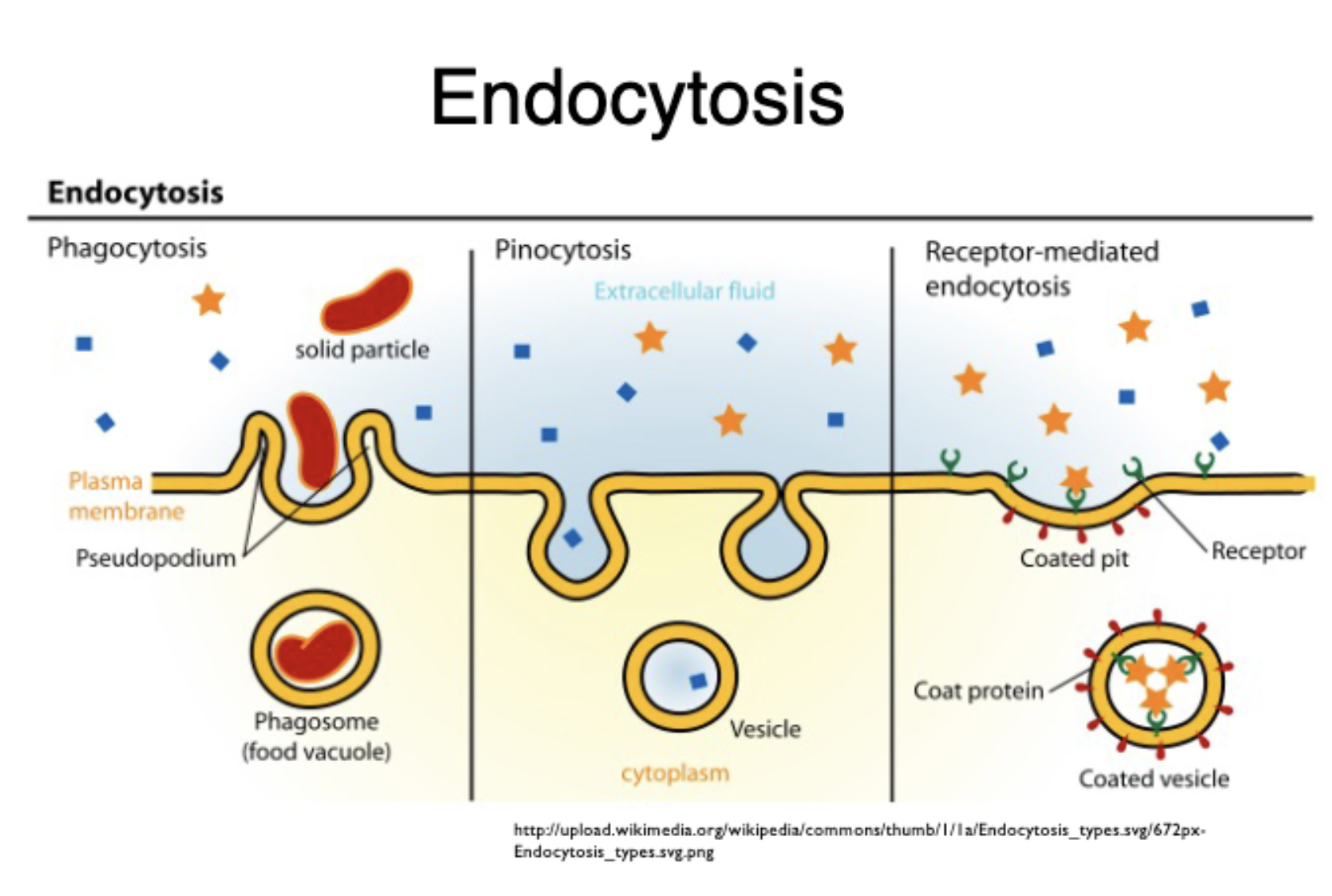
endocytosis
movement of materials inside the cell; mediates via actin
phagocytosis
cell “eating”; movement of a molecule into the cell
pinocytosis
cell “drinking”; movement of fluid/liquid into the cell
receptor mediated endocytosis
receptor proteins on cell surface capture a specific substance
flip over to see images of the types of endocytosis
motor proteins
used to move cargo (vesicles) + binds to microtubules
dynein and kinesin
dynein
go from the + to - end of microtubule with vesicle (periphery to center)
kinesin
go from the - to + end of microtubule with ATP hydrolysis (center to periphery)
energy
the ability to do work
entropy (S)
the disorder of a system
favorable reactions increase entropy (entropy is unusable energy)
free energy (G)
the usable energy that can be manipulated to do work
∆G equation + what is ∆G
∆G = Gproducts - Greactants
∆G is the usable energy released by a reaction; not all the energy released from a reaction is usable however…
first law of thermodynamics
energy is conserved in a reaction — it can be transformed but not created or destroyed
∆E = 0 in reaction
do you always get 100% usable energy out a reaction?
NO! in chemical reaction, you can have lots of potential free energy, but you cannot harvest all of it out of a reaction
Second Law of Thermodynamics
in a closed system, free energy decreases and the amount of unusable energy (aka entropy) increases
what is a way that organisms can harness unusable energy/waste?
when an organism gives off waste/unusable energy, another organism can use it for its own needs
ex. after respiration, we release carbon dioxide, which can be used by plants for their own energy
at what temperature is water most dense? why is that important
most dense at 4 celsius
important bc ice floats above water and cold water sinks (preserves life)
in the reaction A —> B, what happens to the change in energy? ∆S?
∆E = 0 — first law of thermodynamics
∆S > 0 (positive) — second law of thermodynamics
endothermic
a reaction that requires an input of heat energy
exothermic
a reaction that releases heat energy to its surroundings
enthalpy (H)
the total energy in a system
what does it mean when ∆H > 0? ∆H < 0?
∆H > 0 —> reaction absorbs heat, endothermic
∆H < 0 —> reaction releases heat, exothermic
temperature
indicates the direction in which energy flows as heat —> higher to lower temperatures while objects are in thermal contact
interpret ∆H = ∆G + T∆S in words
change in total energy (∆H) = change in usable energy (∆G) + change in unusable energy (T∆S)
how can the free energy be harnessed?
in chemical reactions that break down things (catabolic), energy is released and we can try to harness that (ex. creating ATP)
the energy can be captured and used
exergonic
a reaction that has a negative ∆G + is thermodynamically favorable (spontaneous)
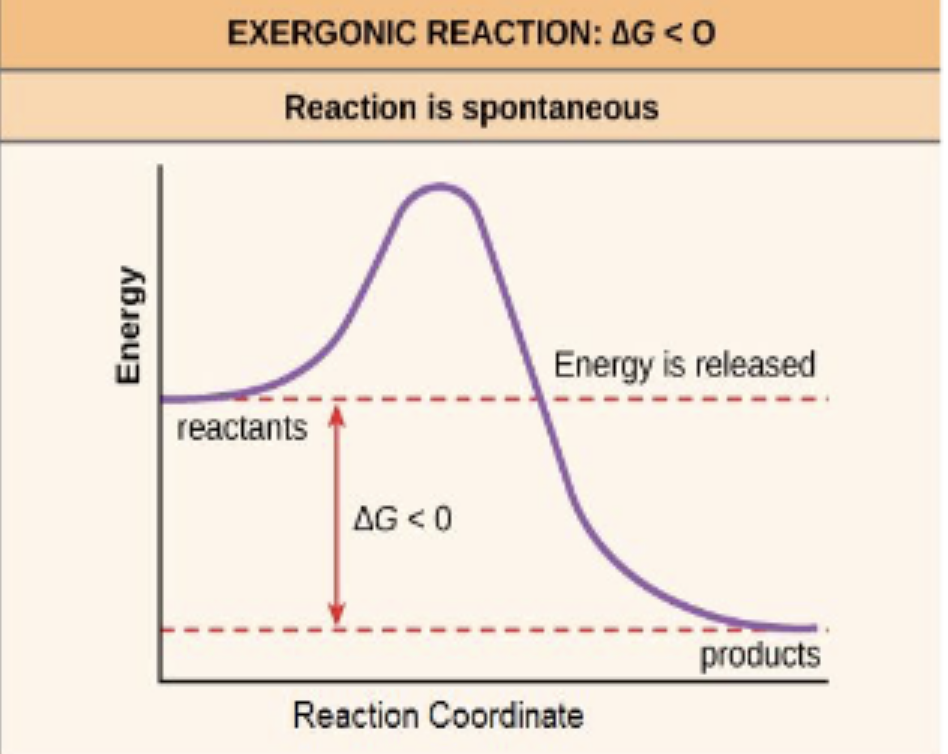
catabolic reaction
a reaction in which a larger molecule is broken down into smaller molecules; often releases energy (exergonic)
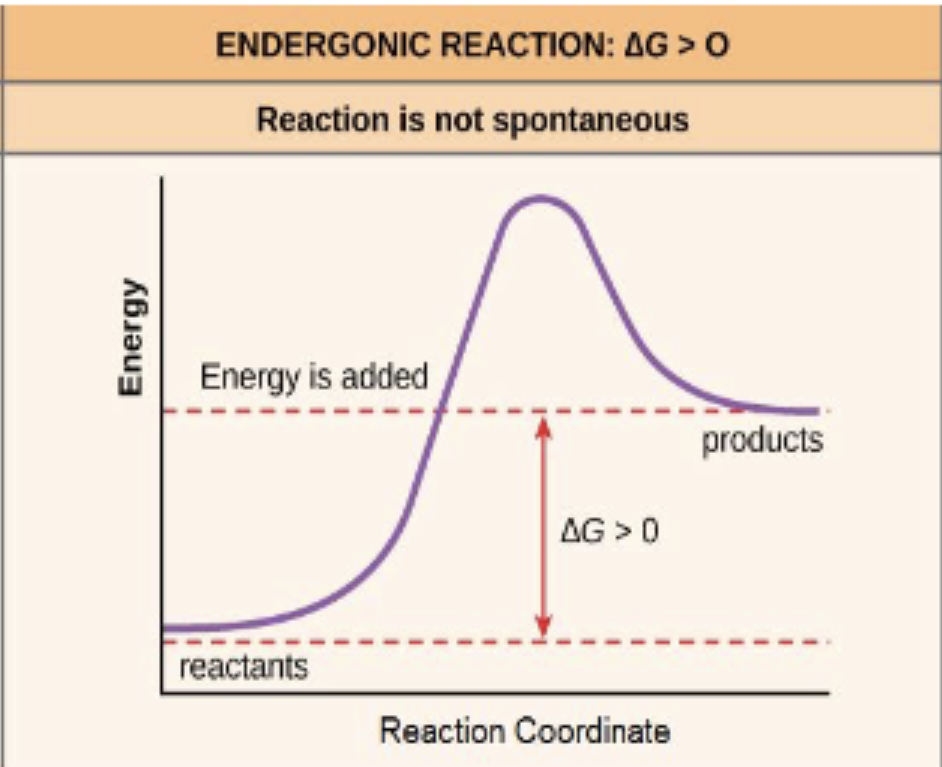
endergonic
a reaction that has a positive ∆G and requires addition of energy (thermodynamically unfavorable/not spontaneous)
free energy of the system increases
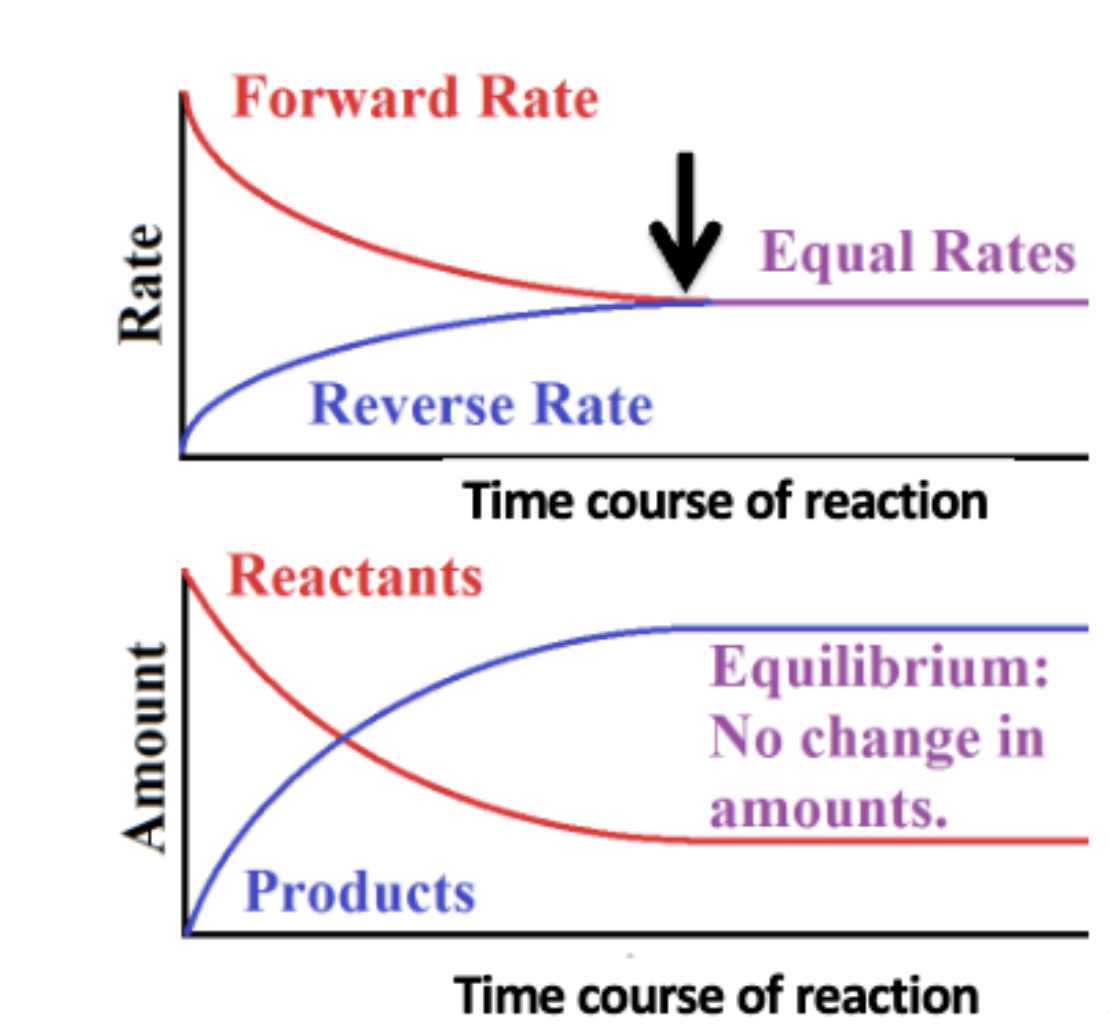
chemical equilibrium
the state in which forward and backward reaction rates are the same and there is no net change in the concentrations of reactants or products
this is because chemical reactions can run in both directions
Keq
the equilibrium constant
Keq = products/reactants
when is Keq > 1? Keq < 1?
Keq > 1 when the reaction is exergonic/spontaneous
Keq < 1 when the reaction in endergonic/not spontaneous
what is ∆G at equilibrium?
0
because forward and backward rxns running at same rate
how can we keep the forward reaction moving?
keeping product concentrations low or keeping reactant concentrations high, since reactions want to reach equilibrium
cells naturally keep product concentrations low (intermediates)
what happens if you remove products/add reactants?
the rate of the forward reaction would increase/the forward reaction would be more favored, to get the system to equilibrium
vice versa applies to removing reactants/adding products
the importance of speed to reactions
even though breakdown is thermodynamically favorable/spontaneous, it still takes time
not all reactions happen at the same rate
ex. decomposition of wood
activation energy
the minimum amount of energy needed to start a reaction (aka energy barrier)
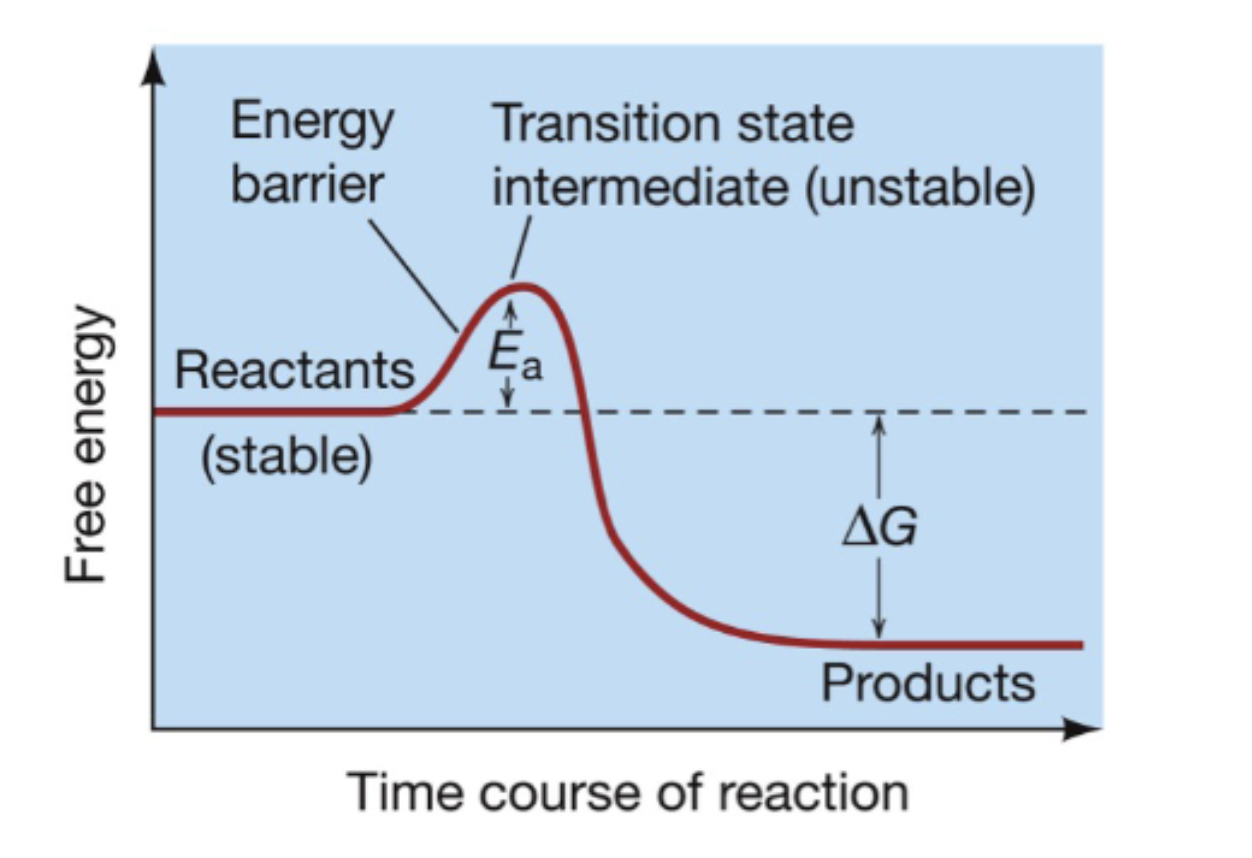
catalysts
substance that speeds up a reaction without being consumed
properties of catalysts
not consumed in the reaction
no catalyst can make a reaction occur that doesn’t already happen
they lower the activation barrier but don’t change ∆G
enzymes (proteins) and ribozymes (RNA) serve as catalysts in biological processes
reaction coupling
when you couple an exergonic reaction (ex. breakdown of ATP) with an endergonic reaction to make the overall process spontaneous
usually use this to drive anabolic processes in a cell
ATP
the acidic anhydrous bonds between the 3rd and the 2nd and the 2nd and the 1st phosphate group hold energy
hydrolyzing these bonds release energy that can be used in reaction coupling (exergonic)
why do cells rely on ATP?
for the capture and transfer of the free energy needed for chemical work
reduction potential (E0’)
tendency of compound to gain electrons and be reduced (intrinsic to each compound)
more positive reduction potential = greater affinity to be reduced/gain electrons
standard reduction potential reaction
2H+ + 2e- = H2, E0’=0.00V at standard conditions
at physiological conditions, has a E0’ = -0.44 (H2 wants to be oxidized)
how to determine if a compound is more inert
more O = more inert
how to determine if a compound has more energy
more H = more energy
reduction
gaining electrons
oxidants/oxidizing agents are reduced
oxidation
losing electrons
reductants/reducing agents are oxidized
in the reaction AH + B+ —> BH + A+, which is the oxidant? reductant?
oxidant: B+
reductant: AH
how to read electron tower/table from top to bottom
if close to the top (more negative reduction potential), will want to lose electrons (an energy source like glucose)
if closer to the bottom (more positive reduction potential), will want to gain electrons (electron acceptors like O2)
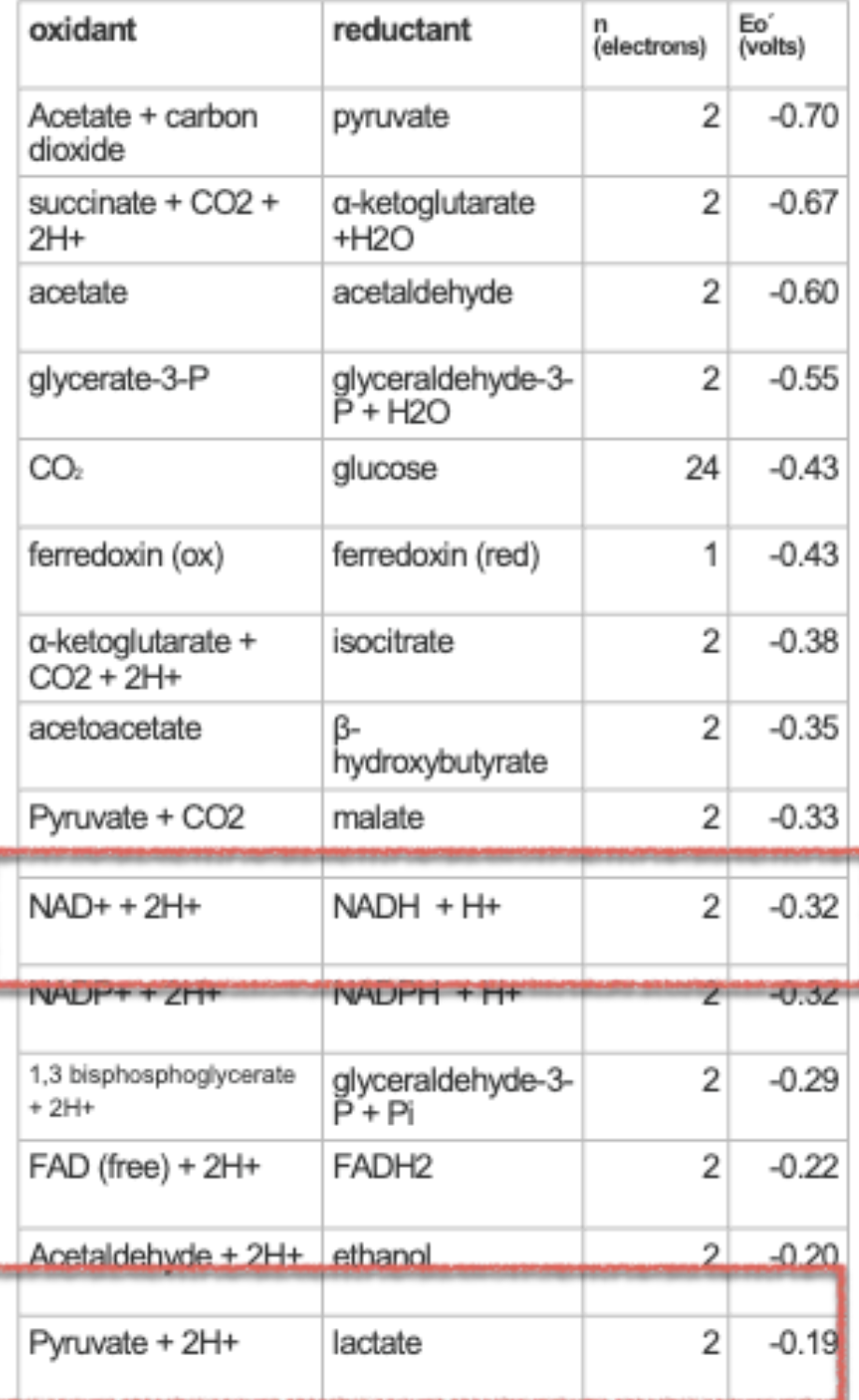
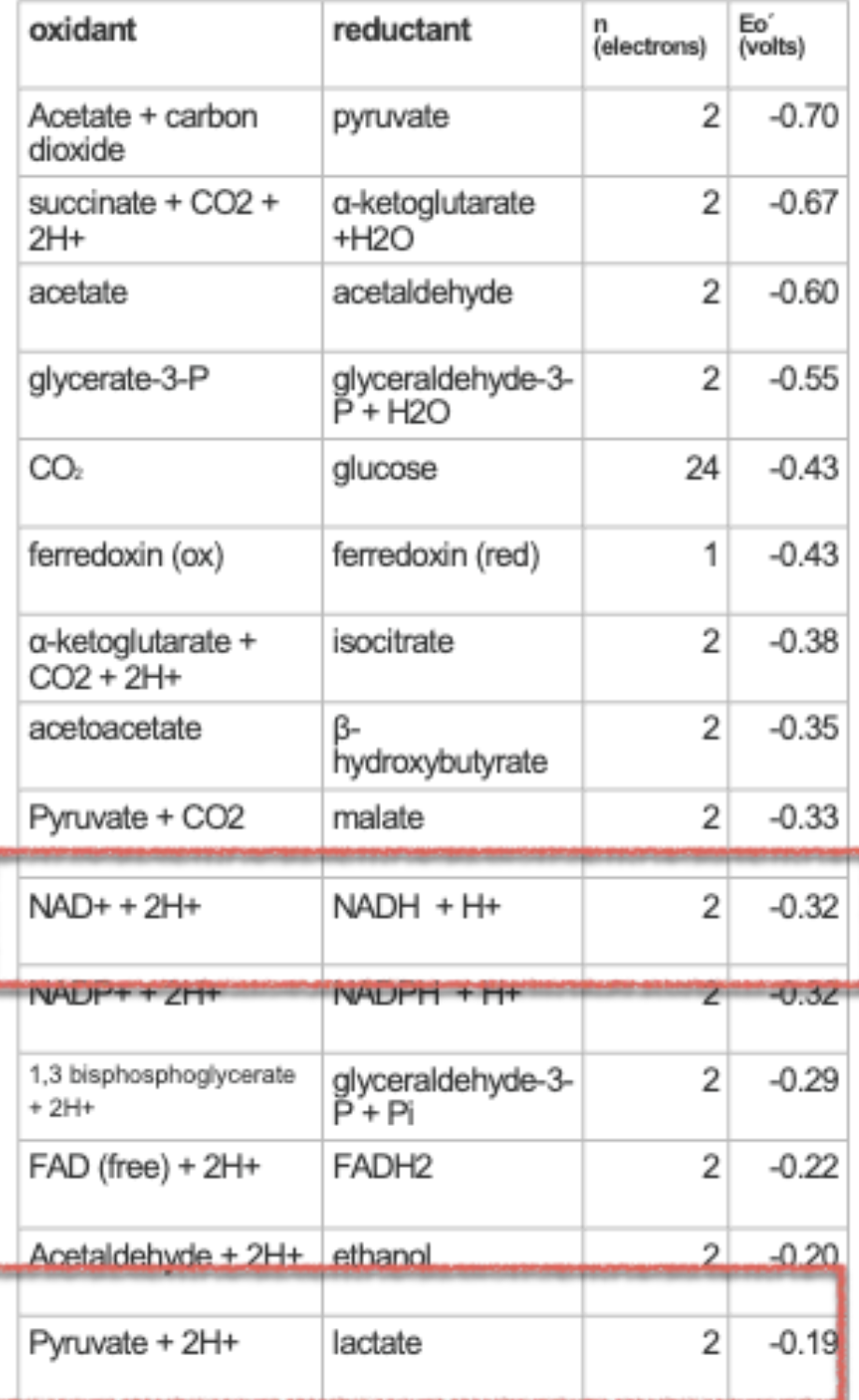
calculating ∆E0’ + what sign should it be
∆E0’ = ∆Eoxidant - ∆Ereductant
this should be positive for it to be spontaneous!!!
relationship between ∆G and ∆E0’
∆G = -nF∆E0’
the sign of ∆G and ∆E0’ should be opposite of each other (inversely related)
how do cells convert food/energy source to energy?
redox reactions! transfer of electrons from a reduced compounds (like glucose) to one that is not
substrate level phosphorylation (SLP)
removing a high energy phosphate from one compound (look for anhydride bond) and putting it on another compound (like ATP)
the newly phosphorylated compound can be used as an energy source!
exergonic (bc creates ATP, which captures free energy)
where does the energy for SLP come from?
from food!
in this case, food is the original source of electrons (which generate energy) and for that high energy phosphate group
glycolysis
10 step process that converts glucose (6 carbon sugar) to 2 pyruvate (3 carbon sugar)
generates 2 net ATP and 2 NADH per glucose
what happens in the first half of glycolysis (aka energy investment phase)?
glucose 6-phosphate is converted into 2 molecules of glyceraldehyde 3-phosphate (G3P)
uses 2 ATP to phosphorylate twice
what happens in the second half of glycolysis (aka energy payoff phase)?
2 G3P are converted into 3 pyruvate molecules
generates 2 ATP and 1 NADH per G3P
key reactions: G3P —> 1,3 bisphosphoglycerate
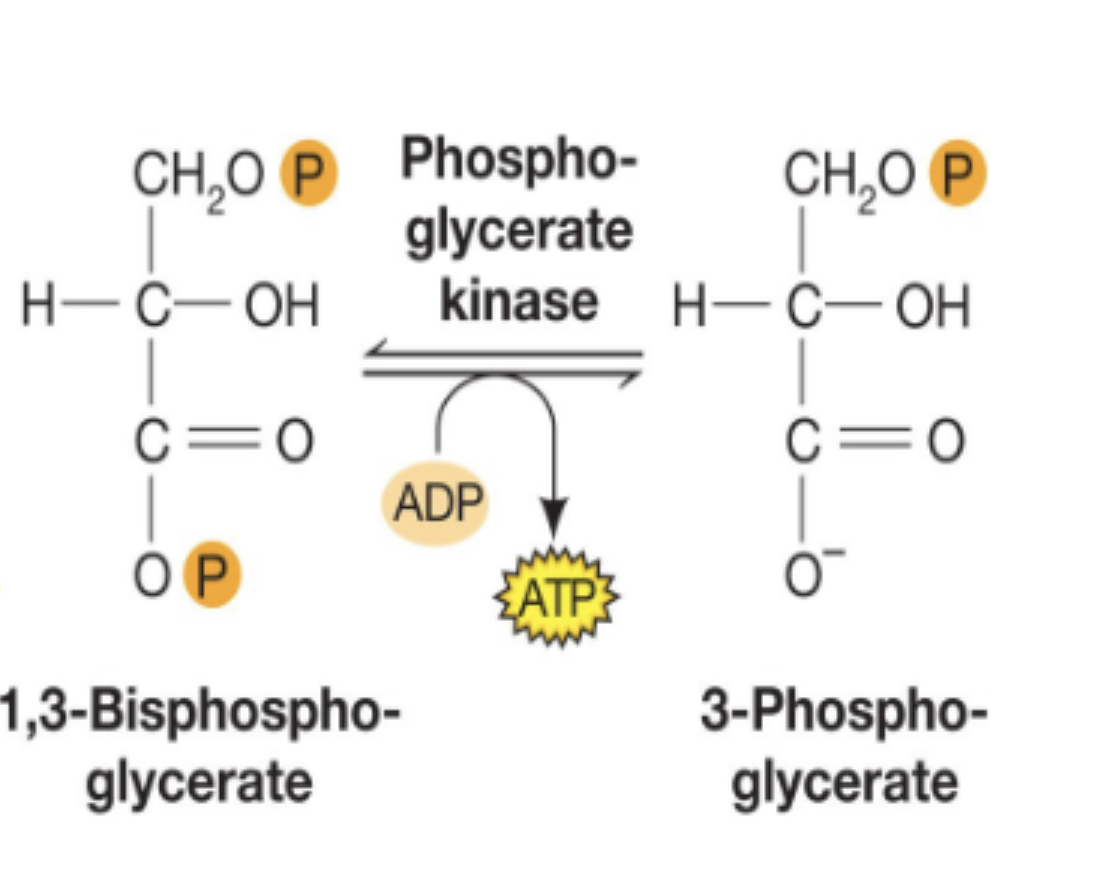
1,3 bisphosphoglycerate —> 3 phosphoglycerate
what happens in G3P to 1,3-bisphosphoglycerate? what key molecule is generated from this process? what type of reaction is this?
G3P (reductant) is converted to 1,3-bisphosphoglycerate (releases electrons)
this process generates 1 NADH molecule (reduced from NAD+ to NADH) and 1 phosphate is added to G3P
this is a redox reaction
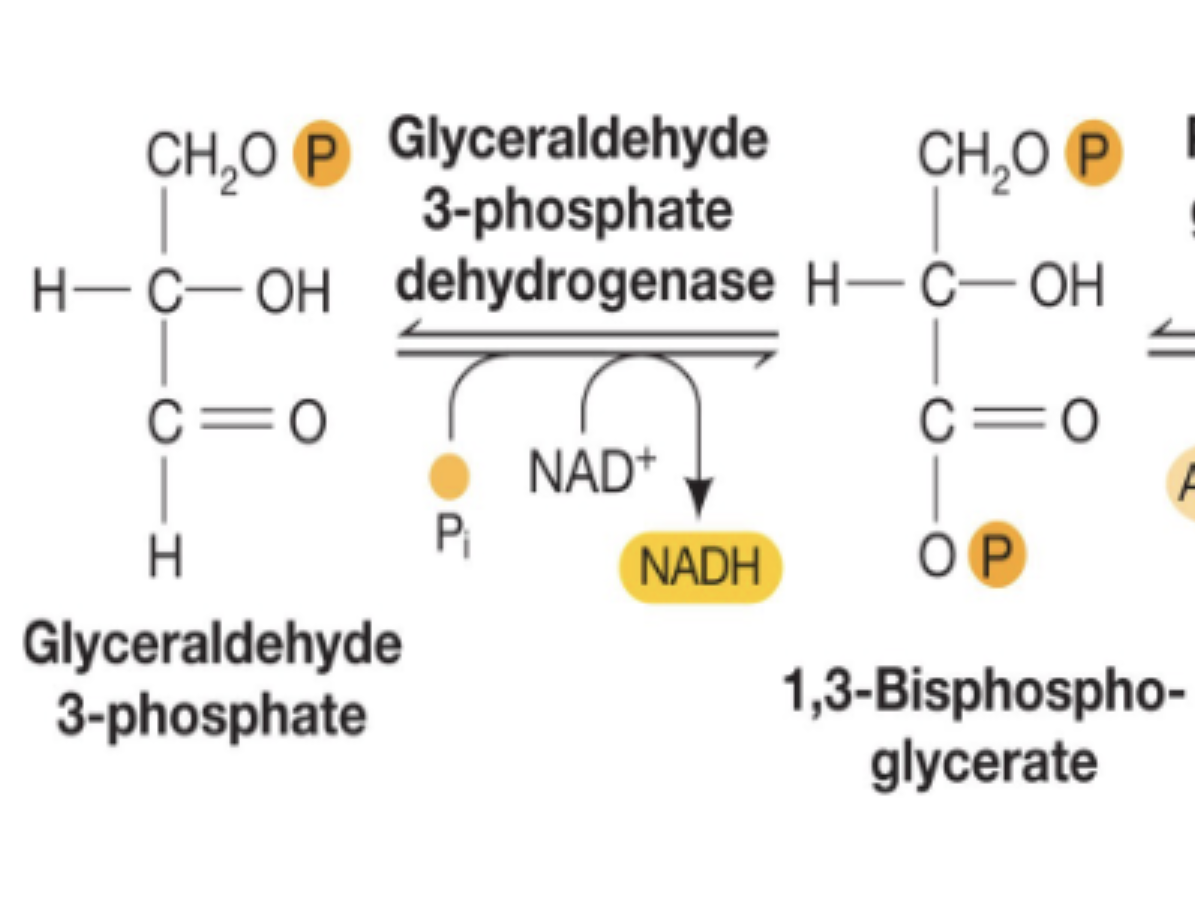
dehydrogenase
an enzyme that is involved in redox reactions; found in G3P to 1,3-bisphosphoglycerate
1,3-bisphosphoglycerate to 3-phosphoglycerate
high energy phosphate (that stored energy from G3P reaction) is transferred from 1,3-bisphospho. onto ADP to create 1 ATP