Change of states
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
Why does heating a system change the energy stored within the system and raise its temperature or produce changes of state?
When heating a system, the energy stored goes towards making the molecules vibrate/ move more, therefore increasing their kinetic energy and causing the temperate to increase or causing them to change state (e.g. solid to liquid, liquid to gas)
What is the relationship between temperature and internal energy?
The higher the temperature, the higher the average kinetic energy of the molecules
they move faster
What can you observe when a body is changing state?
When a body is changing state, the energy stored is used to breaking the forces between the molecules rather than increasing their kinetic energy, therefore the temperature stays constant
Describe the arrangement and motion of particles in solids
Closely packed
Vibrate in fixed positions
high density
Low KE
definite shape and volume
Describe the arrangement and motion of particles in liquids
closely packed
can flow over one another
medium density
medium KE
no definite shape
definite volume
Describe the arrangement and motion of particles in gases
far apart
move randomly
low density
no definite shape
no fixed volume
highly compressible
Describe the changes that occur when a solid melts into a liquid.
For a solid to melt, it must be equal or above its melting point
Thermal energy transfer therefore takes place and supplies the particles in the solid with energy in their kinetic store
This breaks the rigid bonds between the particles, meaning they are able to flow over each other
Describe the changes that occur when a liquid evaporates into a gas.
For the liquid to evaporate or boil, it must be equal or be above its boiling point
Thermal energy transfer therefore takes place and supplies the particles on the surface of the liquid with energy in their kinetic store
This removes the bonds between the particles, meaning they are able to move randomly and are spread far apart
Describe the changes that occur when a liquid boils into a gas.
Gas bubbles are produced within the liquid
As gas is less dense than liquids, they rise to the surface and escape to the surroundings, forming a gas
they do not evaporate from the surface
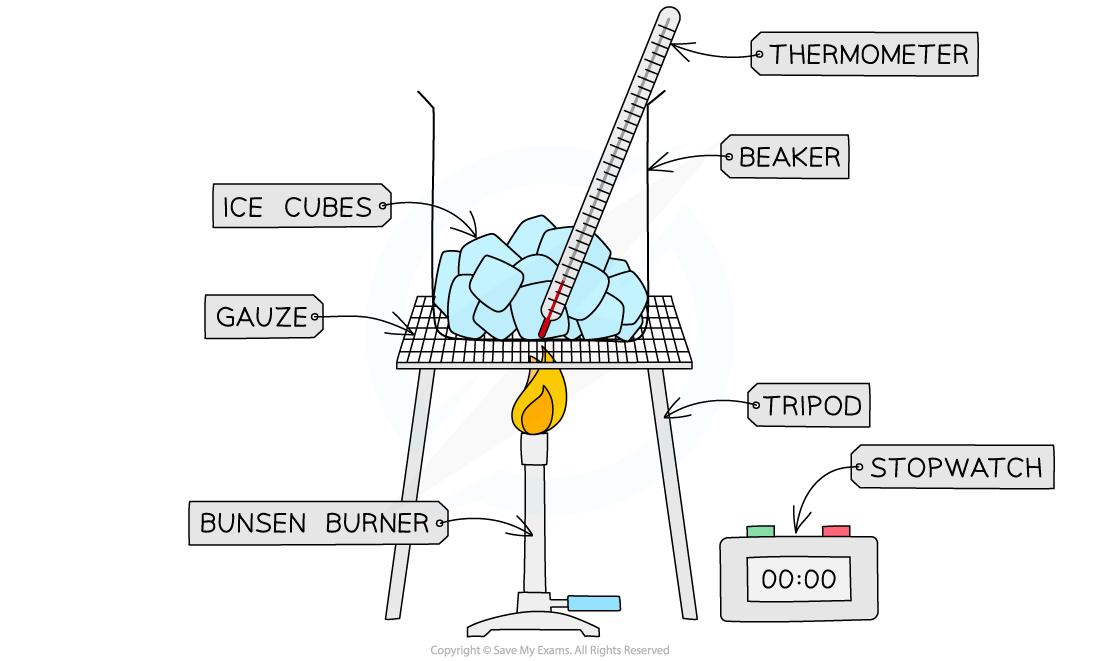
Describe a practical obtaining a temperature-time graph to show the constant temperature during a change of state.
Place the ice cubes in the beaker so that it is roughly half full
Place the thermometer in the beaker
Place the beaker on the tripod and gauze and slowly start heating it using a Bunsen burner
While heating, take temperate measurements at regular intervals (e.g. 1 minute)
Continue this until the ice cubes has fully melted into water
x-axis = time
y- axis = temp.
Should be able to observe that the temperature remains at 0’C while changing states
What are some ways to prevent errors and improve accuracy of experiment?
Measure temp. at eye level
Ensure thermometer is held vertically
Ensure there are enough ice cubes surrounding the thermometer
Only begin experiment when temp. is below 0’C
What is specific heat capacity?
The amount of energy required to change the temperature of 1 kg of a substance by 1’C per kilogram of mass (J/kg’C)
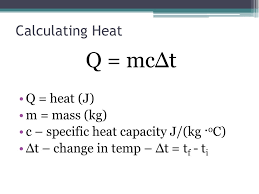
State the formula linking change in thermal energy, mass, specific heat capacity and change in temperature.
change in thermal energy = mass x specific heat capacity x change in temperature
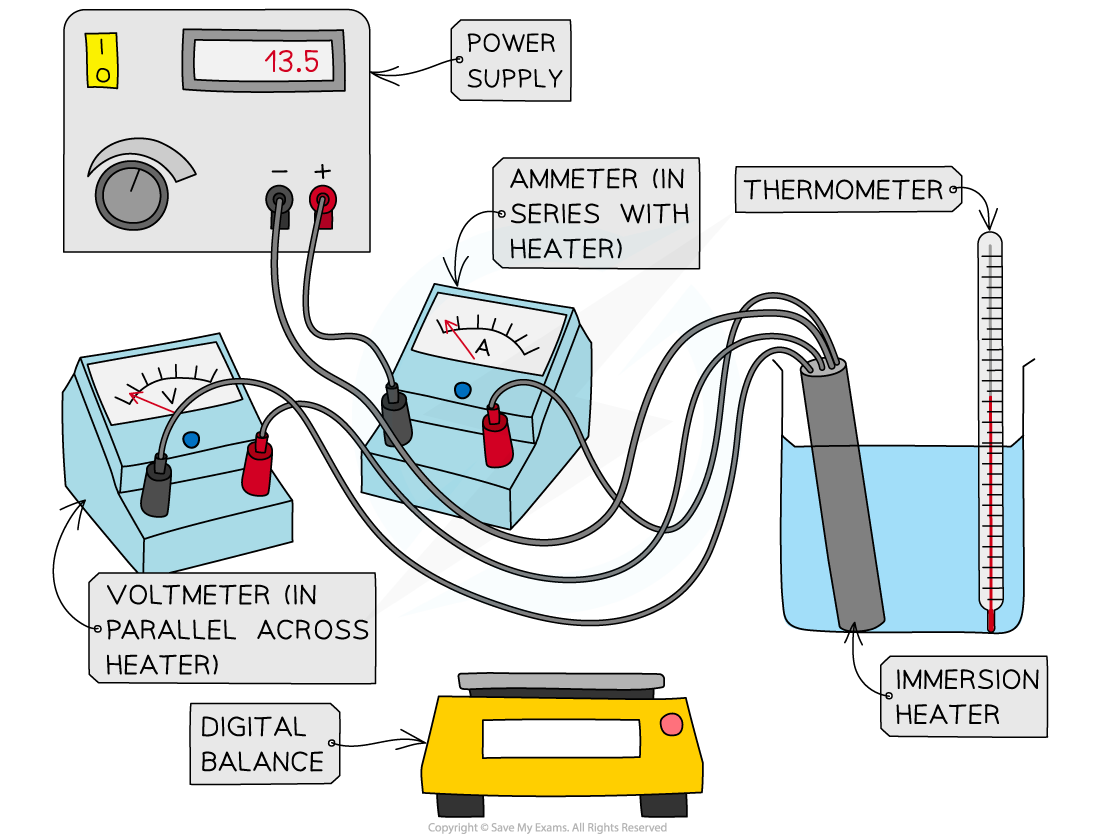
Describe a practical investigating the specific heat capacity of water.
Put a thermometer in to a known mass of water in a beaker
Measure the initial temperature
Put an immersion heater into the beaker, powered by a power supply
Switch the heater on, and start the stopwatch for 5 minutes.
During so, take readings from the ammeter in series) and voltmeter (in parallel) at the start and at the end
Using the formula E = V x I x t, measure the thermal energy
Then at the end of the 10 minutes, record the final temperature to get the change in temperature
Use the formula c = E/ c x ∆T to find the specific heat capacity
(In the case that there is a known amount of thermal energy, skip those steps)
Describe a practical investigating the specific heat capacity of some solids.
Measure the mass of the block using a top pan balance
embed a thermometer and an immersion heater into the block
Measure the initial temperature of the block
Insulate the block by coating it in a layer of sponge or cotton wool to help reduce heat loss
Attach the immersion heater to a power supply, ammeter and voltmeter
Turn on the immersion heater and start the stopwatch for 10 minutes
During that, take readings from the start and finish
Measure the change in thermal energy using E = V x I x t [l = load (amps)]
Measure final temperature to find the change in temperature
Using c = Q/ m x ∆T, find the specific heat capacity
![<ul><li><p>Measure the mass of the block using a top pan balance</p></li><li><p>embed a thermometer and an immersion heater into the block</p></li><li><p>Measure the initial temperature of the block</p></li><li><p>Insulate the block by coating it in a layer of sponge or cotton wool to help reduce heat loss</p></li><li><p>Attach the immersion heater to a power supply, ammeter and voltmeter</p></li><li><p>Turn on the immersion heater and start the stopwatch for 10 minutes</p></li><li><p>During that, take readings from the start and finish</p></li><li><p>Measure the change in thermal energy using E = V x I x t [l = load (amps)]</p></li><li><p>Measure final temperature to find the change in temperature</p></li><li><p>Using c = Q/ m x ∆T, find the specific heat capacity</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/38a5eb3a-fe80-4c06-b794-0b3fef0525e4.png)