Organic Compounds
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
Alkanes
Have the general formula CnH2n+2 where n=1, 2, 3….. _________- only have single bonds. They are also known as saturated hydrocarbons. They are referred to as saturated hydrocarbons because they contain the maximum number of hydrogen atoms that can bond to the carbon atoms present; that is, they are saturated with hydrogen atoms. In naming alkanes, the –_____suffix (ending) is used.
cycloalkane
Alkanes whose carbon atoms are joined in rings are called _________. They have the general formula CnH2n. The simplest __________ is cyclopropane.
Alkenes
Are hydrocarbons that contain at least one carbon-carbon double bond. They are also called olefins. Their formula is CnH2n where n = 2, 3….. _________ are classified as unsaturated hydrocarbons as opposed to the alkanes, which are saturated hydrocarbons. In naming alkenes, the –ene suffix (ending)is used.
They are insoluble in water due to their nonpolar feature, but are more reactive than alkanes.
Alkynes
contain at least one C-C triple bond. They have the general formula CnH2n-2 where n = 2, 3.…
In naming _________, the –yne suffix (ending) is used. The name of the parent compound is determined by the number of carbon atoms in the longest chain.
are more reactive and have slightly higher boiling points compared to alkanes and alkenes. They dissolve in organic solvents, are slightly soluble in polar solvents, and are insoluble in water.
Aromatic Hydrocarbons
Are a class of hydrocarbons whose molecules contain a ring of six carbon atoms (benzyl ring) attached.
Functional Groups
A group of atoms that is largely responsible for the chemical behavior of the parent molecule. Compounds containing the same functional group undergo similar reactions.
Alcohol
Contain the hydroxyl functional group, -OH. Very weakly acidic and are soluble in water because of their polar nature. Are highly flammable.
Ethyl Alcohol
Produced biologically by the fermentation of sugar or starch, is by far the best known.
Carboxulic Acids
These acids are weak in nature and are widely found in both plant and animal kingdoms.
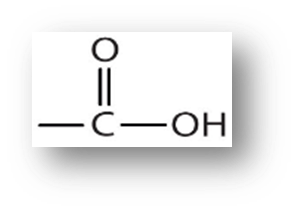
Acetic Acid
One of the common carboxylic acids, is also known as vinegar.
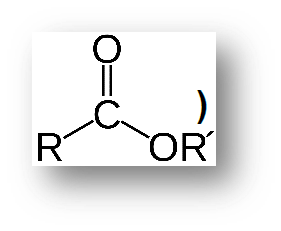
Esters
Hhave the general formula R’-COOR, where R’ can be a hydrocarbon group or Hydrogen and R is a hydrocarbon group. They are used in the production of perfumes and as flavoring agents. The smell and flavor of many fruits come from the presence of small quantities of ____——-. are polar and water-soluble.
Amines/Amides
are organic bases having the general formula R3N, where R may be Hydrogen or a hydrocarbon group. When amines are allowed to react with acids, they form colorless and odorless salts. When all Rs are hydrogens, the resulting compound is ammonia, NH3.