Ch7 Cellular Respiration
1/78
Earn XP
Description and Tags
A comprehensive set of vocabulary flashcards covering the major concepts, molecules, enzymes, pathways, carriers, and regulatory mechanisms involved in cellular respiration, glycolysis, the citric acid cycle, oxidative phosphorylation, fermentation, and related metabolic processes.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
79 Terms
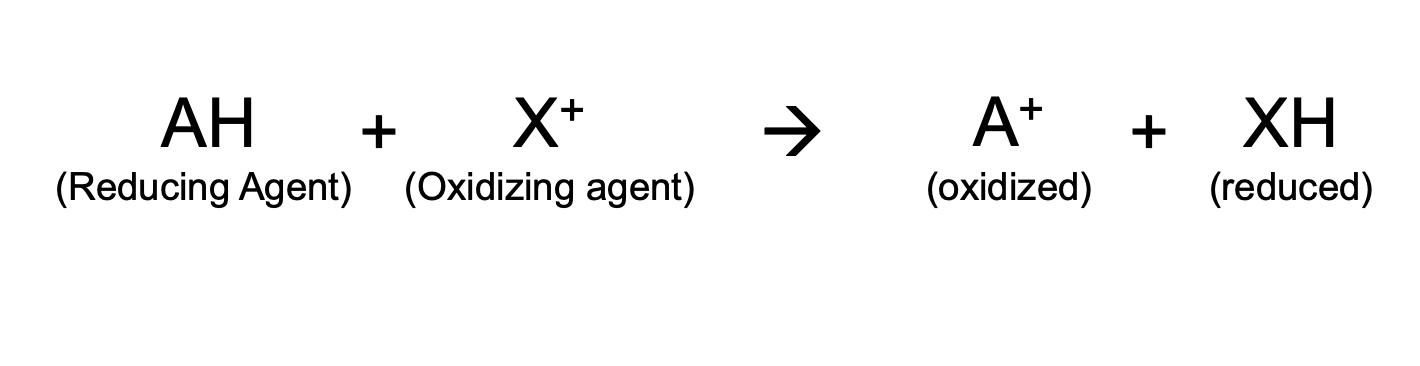
Redox reaction
Consist of oxidation (loss of electrons) and reduction (gain of electrons) that usually happen simultaneously.
Importance of Electrons
serve as carriers of energy for cellular functions.
reducing agents
Molecules that can donate electron(s) in a redox reaction
oxidizing agents
those that can accept electrons
Oxidation
-Loss of electrons by a molecule; often releases energy; the oxidized form is the donor of electrons.
loss of electrons → loss of potential energy.
Reduction
Gain of electrons by a molecule; the reduced form accepts electrons.
gain of electrons → gain of potential energy.
High-energy electrons are crucial for energy transfer within the cell.
Electron carrier
a molecule that transports electrons during cellular reactions, helping transfer energy from one molecule to another.
they bind and carry high-energy electrons between compounds in biochemical pathways.
(e.g., NAD+, NADH, FAD, FADH2, NADP+, NADPH).
Nicotinamide adenine dinucleotide (NAD+)
A vitamin-derived coenzyme that accepts electrons to become NADH; a key electron carrier.
Formed from vitamin B3,
NAD+: Oxidized form
NADH: Reduced form after accepting two electrons and a proton.
Reaction: RH + NAD+ → NADH + R
NADH
Reduced form of NAD+; carries high-energy electrons to the electron transport chain.
NADP+/NADPH
NADP+ with an extra phosphate; NADPH is the reduced form used in anabolic reactions and photosynthesis.
FADH2
Reduced form of FAD; donates electrons to the electron transport chain at a lower yield than NADH.
NADP
NADP: Variation of NAD with an extra phosphate, plays a role in anabolic reactions.
Adenosine triphosphate (ATP)
Energy currency of the cell; energy stored in its high-energy phosphate bonds and released by hydrolysis.
Cells cannot store large amounts of free energy → too much heat would damage proteins.
ATP acts as a “rechargeable battery” providing on-demand energy. where energy is released upon breaking down ATP to ADP.
Cellular Respiration
Glucose + O₂ → CO₂ + H₂O + ATP
the process by which cells break down glucose and other molecules to produce ATP, the main energy source for the cell.
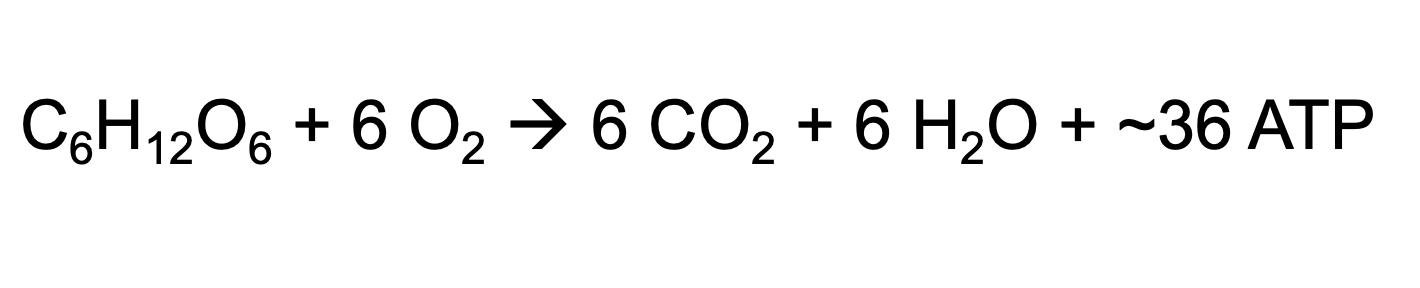
Metabolic pathways involved in Cellular Respiration
Glycolysis
Oxidation of Pyruvate and Citric Acid Cycle (Krebbs cycle/TCA)
Oxidative Phosphorylation (Electron transport chain, protein synthase)
Together, they break down glucose to release energy and produce ATP for the cell.
Hydrolysis of ATP
ATP → ADP + Pi + Energy.
the process of breaking down ATP (adenosine triphosphate) into ADP and a phosphate group, releasing energy that the cell can use for its activities.
exergonic and can provide energy for a coupled endergonic reaction.
Structure of ATP
Comprised of adenosine monophosphate (AMP), ribose sugar, and three phosphate groups.
Negative phosphate groups repel each other → ATP bonds are high-energy but unstable.
allowing for energy release when bonds are broken.
Adenosine diphosphate (ADP)
Product of ATP hydrolysis; substrate for ATP synthesis.
Adenosine monophosphate (AMP)
Nucleotide form; can participate in energy transfer and signaling; related to ATP/ADP balance.
Phosphorylation
Addition of a phosphate group to a molecule, often regulating activity or energy transfer.
adding a phosphate to ADP to form ATP; requires energy.
tend to be less stable and more likely to react.
dephosphorylation
-The loss of a phosphate group from a molecule
-The release of one or two phosphate groups from ATP
-releases energy.
Substrate-level phosphorylation
Direct transfer of a phosphate group from a substrate to ADP to form ATP.
location: Cytoplasm (glycolysis), mitochondrial matrix (citric acid cycle)
Oxidative phosphorylation
ATP synthesis powered by the transfer of electrons through the electron transport chain and chemiosmosis (proton gradient)
where energy from electrons is used to make large amounts of ATP through the electron transport chain and ATP synthase in the mitochondria.
location: Inner mitochondrial membrane
final stage of cellular respiration
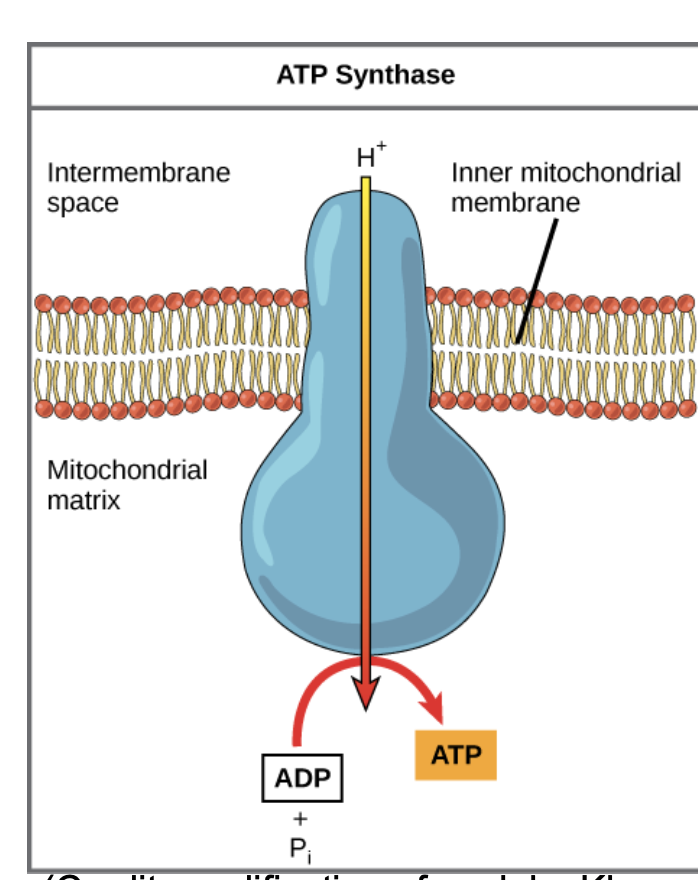
Chemiosmosis
Movement of ions (H+) across a membrane down their gradient to power ATP synthesis.
which produces ATP during cellular respiration or photosynthesis.
Proton gradient
Unequal distribution of protons (H+) across a membrane that powers ATP synthesis.
ATP synthase
enzyme that uses the energy from moving hydrogen ions (H⁺) across a membrane to produce ATP from ADP and phosphate.
Mitochondria
-Organelle where the citric acid cycle and oxidative phosphorylation occur in eukaryotes.
-“powerhouses” of the cell because they produce ATP through cellular respiration, providing energy for the cell’s activities.
Intermembrane space
Space between the inner and outer mitochondrial membranes where protons accumulate.
Matrix
Innermost compartment of the mitochondrion where the citric acid cycle occurs.
Glycolysis (“sugar splitting”)
(no O₂ required) anaerobic breakdown of glucose occurring in the cytoplasm.
first metabolic pathway of glucose metabolism; includes 10 enzymatic reactions.
• Inputs are 1 Glucose, 2 NAD+, 2 ATP, 4 ADP
• Outputs are 2 Pyruvate, 2 NADH, 4 ATP, 2 ADP
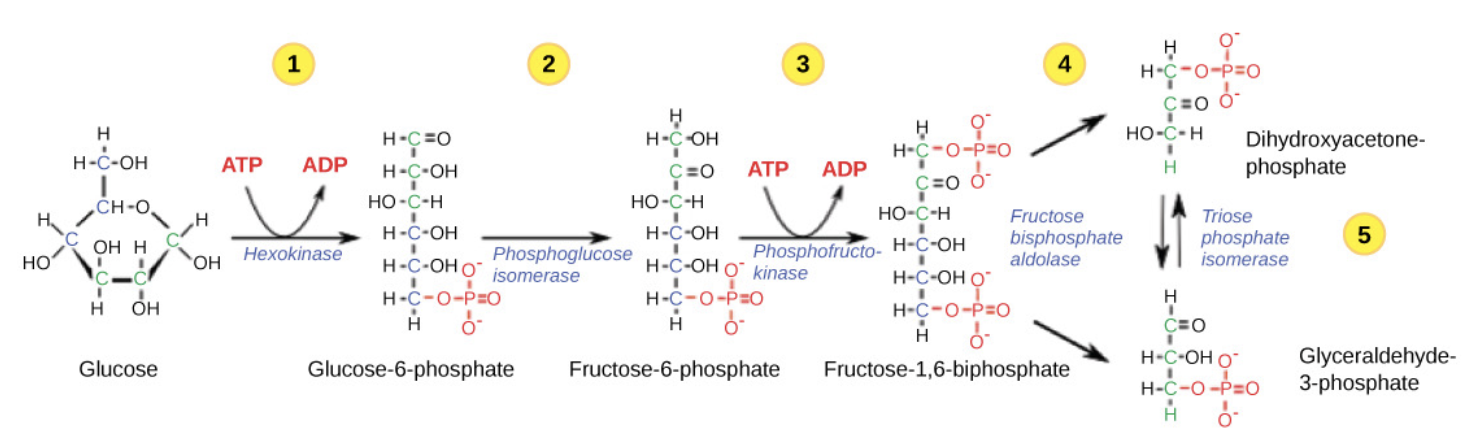
Frist half of Glycolysis
Start: 1 glucose (C6H12O6) → 2 pyruvate (C3H4O3).
Energy Investment Phase: Requires the input of ATP to initiate glycolysis.
involves 5 enzymes and uses two ATP molecules.
• Glucose is phosphorylated twice and then split into two three-carbon molecules, called glyceraldehyde-3- phosphate (G3P).
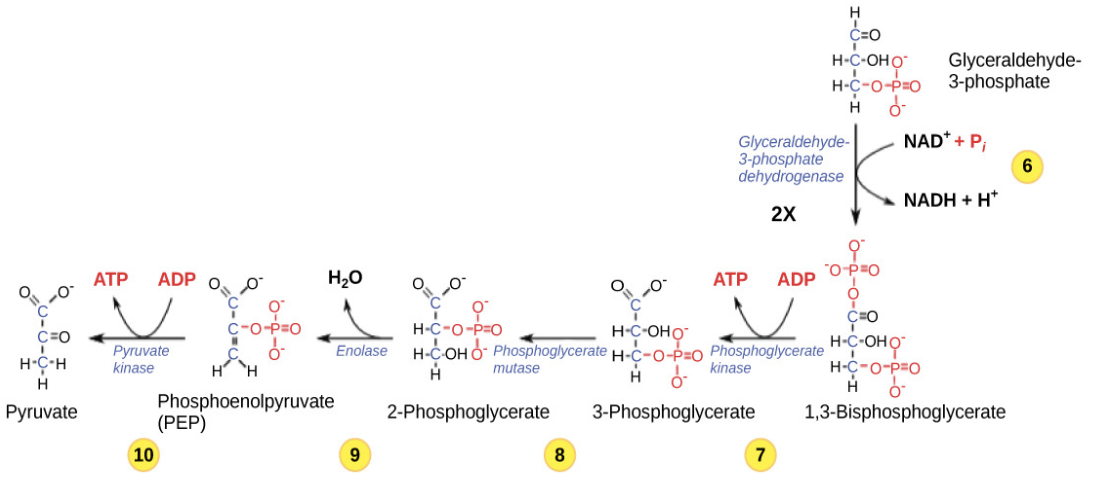
Second half of Glycolysis
Involves energy extraction, yielding ATP and NADH.
involves phosphorylation without ATP investment (step 6) and produces two NADH and four ATP molecules to generate 2 pyruvate molecules
Hexokinase
Glycolytic enzyme that phosphorylates glucose to glucose-6-phosphate using one ATP.
Glucose-6-phosphate
Phosphorylated glucose that cannot easily exit the cell; beginning glycolysis.
Isomerase
Enzyme that converts a molecule into its isomer (e.g., glucose-6-phosphate to fructose-6-phosphate).
Phosphofructokinase
Rate-limiting glycolytic enzyme that phosphorylates fructose-6-phosphate; inhibited by high ATP, citrate; activated by ADP.
Fructose-1,6-bisphosphate
Highly phosphorylated intermediate split into two three-carbon molecules in glycolysis.
Aldolase
Glycolytic enzyme that cleaves fructose-1,6-bisphosphate into DHAP and G3P.
Dihydroxyacetone phosphate (DHAP)
Three-carbon sugar isomer that is converted to glyceraldehyde-3-phosphate in glycolysis.
Glyceraldehyde-3-phosphate (G3P)
Three-carbon sugar that continues in glycolysis and is oxidized to form NADH and ATP.
NAD+ in glycolysis
Electron acceptor in glycolysis; becomes NADH during oxidation of G3P.
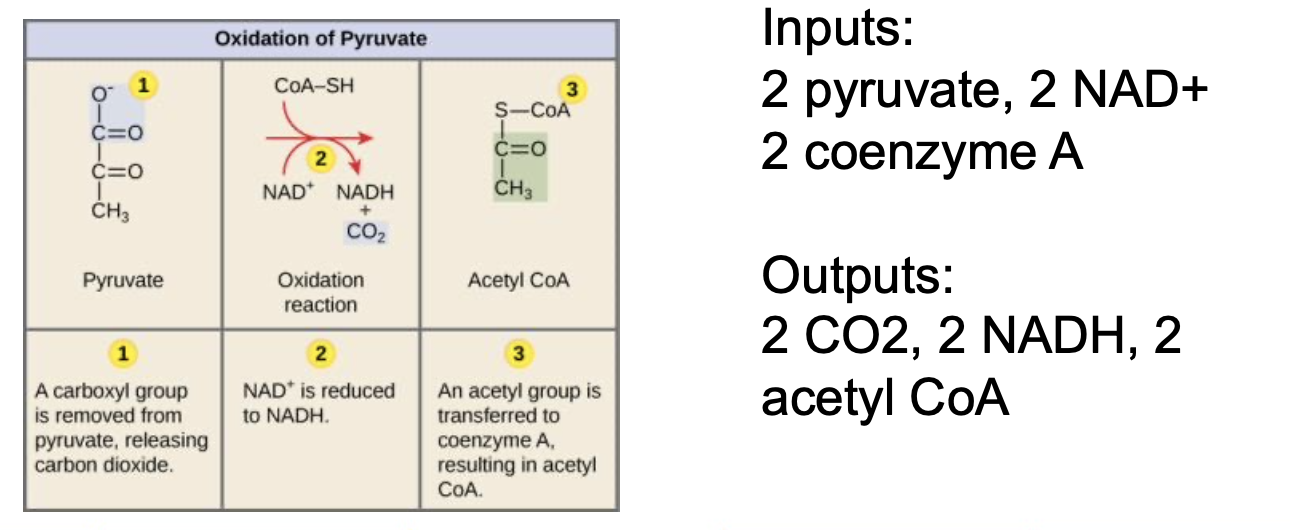
Oxidation Pyruvate (Acetyl CoA)
Occurs in mitochondrial matrix (eukaryotes).
Transformed into acetyl CoA, removing a carbon as CO2.
Consist of 3 steps
Steps (per pyruvate):
Remove CO₂ → 2-carbon fragment remains.
Oxidize fragment; reduce NAD⁺ → NADH.
Attach Coenzyme A → Acetyl CoA (2 C).
Per glucose: 2 CO₂ + 2 NADH + 2 acetyl CoA.
Pyruvate
End product of glycolysis; can be metabolized aerobically to acetyl-CoA or fermented anaerobically.
Pyruvate dehydrogenase
enzyme that converts pyruvate into acetyl-CoA, linking glycolysis to the citric acid cycle and releasing carbon dioxide in the process.
Acetyl-CoA
a molecule formed from the breakdown of pyruvate that enters the citric acid cycle to help produce energy (ATP) for the cell.
Coenzyme A (CoA)
molecule that helps enzymes carry and transfer acetyl groups during cellular respiration, playing a key role in energy production.
Carrier of acetyl groups; derived from pantothenic acid (vitamin B5).
Citric acid cycle / Krebs cycle / TCA cycle
-Occurs in mitochondrial matrix.
-a series of chemical reactions in the mitochondria that break down acetyl-CoA to produce ATP, NADH, FADH₂, and carbon dioxide.
Completes glucose oxidation
Energy Extraction: Through multiple steps producing two CO2, three NADH, and one FADH2 per acetyl CoA.
Oxidative Phosphorylation
Takes place across the inner mitochondrial membrane.
Most of the ATP produced during cellular respiration occurs in the last pathway
only pathway where O2 is an input.
consists of an electron transport chain and chemiosmosis, which generates ATP
Electron Transport Chain (ETC)
A series of protein complexes that transfer electrons to oxygen, pumping protons across a membrane.
a series of proteins in the inner mitochondrial membrane that transfer electrons from NADH and FADH₂, releasing energy to create a proton gradient used to make ATP.
last component of aerobic respiration and is the only part of glucose metabolism that uses atmospheric oxygen.
-provides the energy to power chemiosmosis.
• In the process, protons (H+) are pumped from the mitochondrial matrix to the intermembrane space, and O2 is reduced to form H2O.
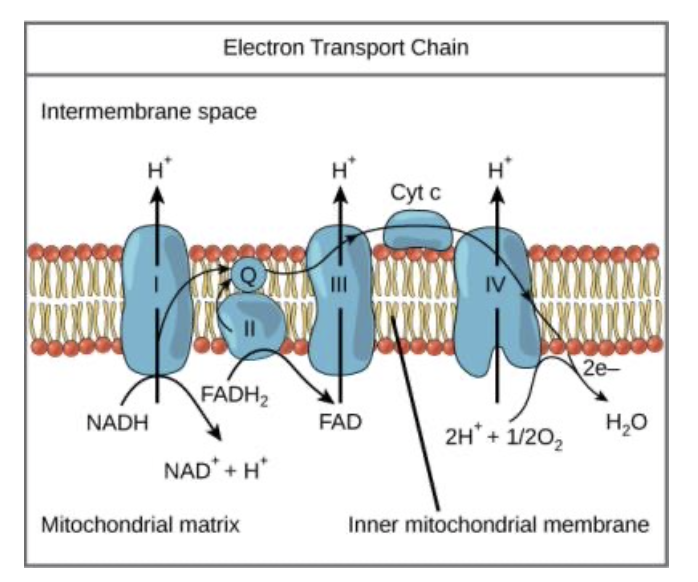
Chemiosmosis
process where hydrogen ions (H⁺) flow through the enzyme ATP synthase across a membrane, using the kinetic energy from the proton gradient to form ATP from ADP and phosphate (Pi).
ATP Yield
The number of ATP generated by cellular respiration is 30-36 per glucose
• Varies by species and how efficiently NADH from glycolysis enters mitochondria
Feedback Inhibition
control of metabolic pathways, such as glycolysis and the citric acid cycle, through allosteric regulation.
It occurs when the final product of a pathway binds to an enzyme involved earlier in the process, reducing its activity to prevent the cell from producing excess amounts of that product.
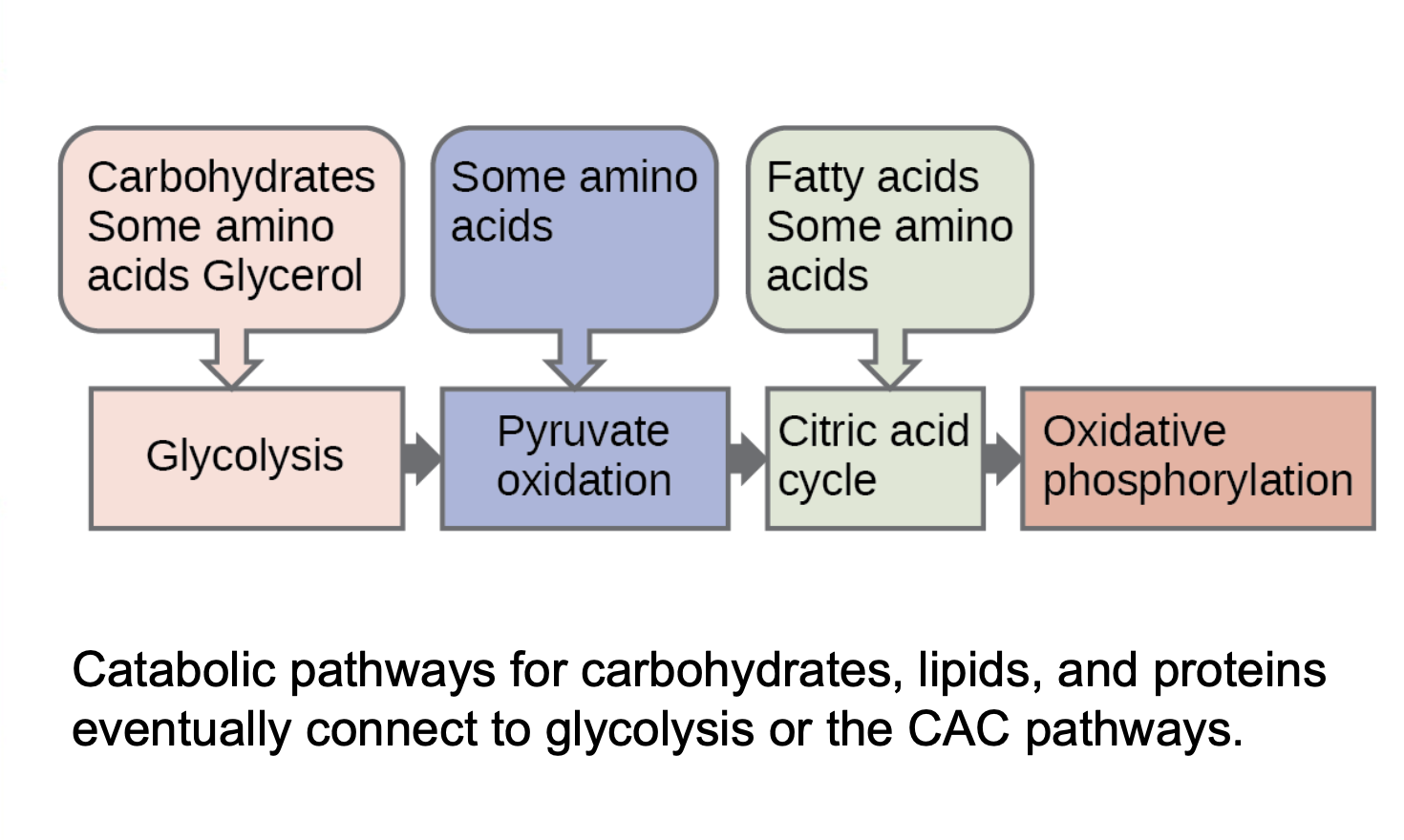
Integration of Pathways
Metabolism of proteins, fats, and carbohydrates contribute toward glycolytic and citric acid cycle pathways.
Both carbohydrates and proteins can enter into glucose catabolism, showing flexibility in metabolic processes.
NADH and FADH2 as carriers
Reduced electron carriers produced in CAC; feed electrons to the ETC.
Complex II (succinate dehydrogenase)
ETC complex that accepts electrons from FADH2 and passes them to ubiquinone, bypassing Complex I.
Complex III (cytochrome bc1)
ETC complex transferring electrons to cytochrome c and pumping protons.
Complex IV (cytochrome c oxidase)
ETC complex that transfers electrons to oxygen and pumps protons; reduces O2 to water.
Oxygen (O2) as final electron acceptor
Final electron acceptor in aerobic respiration, forming water.
What types of environment does glycolysis occurs in?
aerobic and anaerobic environments.
When O2 is lacking, fermentation regenerates NAD+; otherwise, glycolysis would halt.
Anaerobic Cellular Respiration
A form of respiration that occurs in the absence of oxygen, using electron acceptors other than O2 to generate energy.
aerobic Cellular Respiration
respiration that requires oxygen
two common types of fermentation
-Regenerates NAD⁺ so glycolysis can continue without O₂.
• Lactic acid fermentation
• Alcohol fermentation
Lactic acid fermentation
-Anaerobic pathway (process that allows cells to make ATP without oxygen) converting pyruvate to lactate, regenerating NAD+ for glycolysis.
-Pyruvate + NADH ↔ Lactic acid + NAD⁺
Used by muscle cells and bacteria (e.g., yogurt).
Lactate dehydrogenase (LDH)
enzyme that converts pyruvate into lactate during lactic acid fermentation and regenerates NAD⁺ so glycolysis can keep producing ATP.
Alcohol fermentation
anaerobic pathway in which cells make ATP without oxygen by converting pyruvate into carbon dioxide and acetaldehyde, then into ethanol. This process regenerates NAD⁺, allowing glycolysis to continue producing ATP.
Used by yeast to make beer and wine.
Pyruvate → CO₂ + Acetaldehyde; Acetaldehyde + NADH → Ethanol + NAD⁺
Involves two reactions
• First, catalyzed by pyruvate decarboxylase
• Second, by alcohol dehydrogenase
CONNECTIONS OF LIPID AND GLUCOSE METABOLISMS

REGULATION OF CELLULAR RESPIRATION
-Maintain energy homeostasis by feedback inhibition and enzyme control.
-regulated by many mechanisms, including
- Hormonal control of glucose entry into the cell
- Enzyme reversibility (functioning to substrate product equilibrium) or irreversibility (able to exceed equilibrium)
- Enzyme sensitivity to pH changes due to lactic acid build- up
- Feedback controls
Pyruvate decarboxylase
Enzyme catalyzing the first step of alcoholic fermentation (pyruvate to acetaldehyde) with CO2 release.
(enzyme that removes carbon dioxide (CO₂) from pyruvate during alcohol fermentation, producing acetaldehyde as an intermediate step.)
Thiamine pyrophosphate (TPP)
Coenzyme derived from vitamin B1 required by pyruvate decarboxylase.
Alcohol dehydrogenase
Enzyme that reduces acetaldehyde to ethanol, regenerating NAD+.
Mitochondrial disease
Genetic disorders impairing oxidative phosphorylation and energy production.
Beta-oxidation
Fatty acid catabolism in mitochondria yielding acetyl-CoA units.
Glycogen
Polymer of glucose used for short-term energy storage in liver and muscle.
Glycogenolysis
Breakdown of glycogen to glucose-1-phosphate to feed glycolysis.
Glycolysis regulation (key steps)
Regulation mainly at hexokinase, phosphofructokinase, and pyruvate kinase, controlled by ATP/ADP, citrate, and pH.
Fermentation vs anaerobic respiration
Fermentation regenerates NAD+ without using an electron transport chain; anaerobic respiration uses an inorganic final electron acceptor other than O2.
Regulation by ATP/ADP/AMP
Allosteric control of enzymes where energy charge increases or decreases pathway flux.
Interplay with photosynthesis (NADPH/NADP+)
NADP+/NADPH function in anabolic reactions and the light reactions of photosynthesis.
Oxygen delivery and proton motive force
Oxygen enables complete electron transport; proton gradient drives ATP synthesis.