IGCSE Biology: Enzymes
A catalyst alters the rate of reaction without being changed itself. It lowers the activation energy needed to start a reaction.
Every metabolic reaction is controlled by catalysts called enzymes found in any living organism. Without enzymes, reactions would take place very slowly or not at all. They make sure the rate of reactions are great enough to sustain life.
Different enzymes are needed to digest different foods.
- starch - amylase → maltose
- protein - protease → amino acids
- fats - lipase → fatty acids and glycerol
Other enzymes make bigger molecules from smaller molecules. For example, glucose turns into starch by the enzyme phosphorylase.
Enzymes are named according to the reaction they catalyse and ase -ase to the end. For example:
- carbohydrate → carbohydrase
- protein → protease
- fats (lipids) → lipase
- maltose → maltase
- sucrose → sucrase
Enzyme Mode of Action
The active site is a region of an enzyme that has a complementary shape that the substrate will fit into. A substrate is a substance present at the beginning of a reaction. The product is the substance created by the reaction.
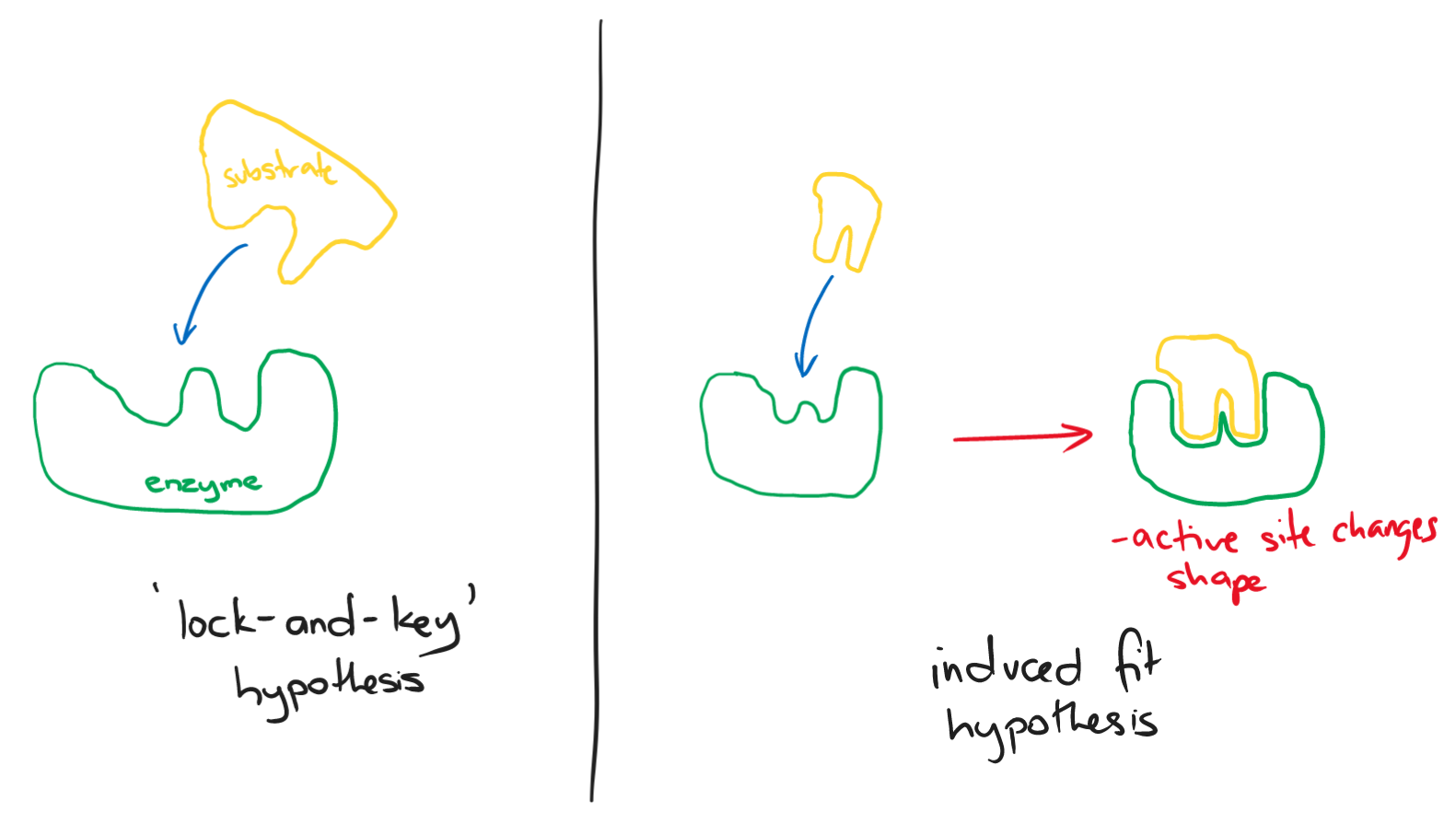
All enzymes have active sites. Each enzyme has an active site that exactly fits into its substrate. This means that each enzyme can only act on a particular kind of substrate. This is called the ‘lock-and-key‘ mechanism.
Another mechanism that is more widely accepted nowadays is known as the ‘induced fit‘ hypothesis
- This mechanism believes the substrate doesn’t fit completely into the active site but has a similar shape
- When the substrate binds to the active site, the active site changes shape to better fit the substrate
Properties of enzymes:
- all enzymes are proteins
- enzymes are made inactive by high temperatures. As protein molecules, they are damaged by heat
- enzymes work best at a particular temperature. Enzymes in the human body work best at 37 degrees Celsius
- enzymes work best at a particular pH. Some enzymes work best in acid (low pH, 1-6); others work best in neutral to alkaline conditions (high pH, 7-14)
- enzymes are catalysts. They are not changed in the chemical reactions they control and can be used over and over again
- ‘lock-and-key‘ mechanism: enzymes are specific to one type of substrate
Factors that Affect Enzyme Activity
- temperature
- Most chemical reactions happen faster at higher temperatures
- Based on collision theory, molecules collide more often as they have more kinetic energy
- collision theory states that, for a reaction to occur, molecules must collide with sufficient energy with the correct orientation
- However, there is an optimal temperature at which enzymes react
- Beyond optimal temperature, the enzymes lose their shape and denature; below the optimal temperature, enzymes don’t collide fast enough and the reaction happens slowly or not at all
- pH level
- The pH of a solution affects the shape of an enzyme
- Most enzymes are at their correct shape at neutral pH of 7
- Enzymes have an optimal pH (just like temperature) at which enzymes react
- Beyond and below the optimal pH, the enzyme will lose their shape and denature