Ch. 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy
1/10
Earn XP
Description and Tags
CHEM334
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
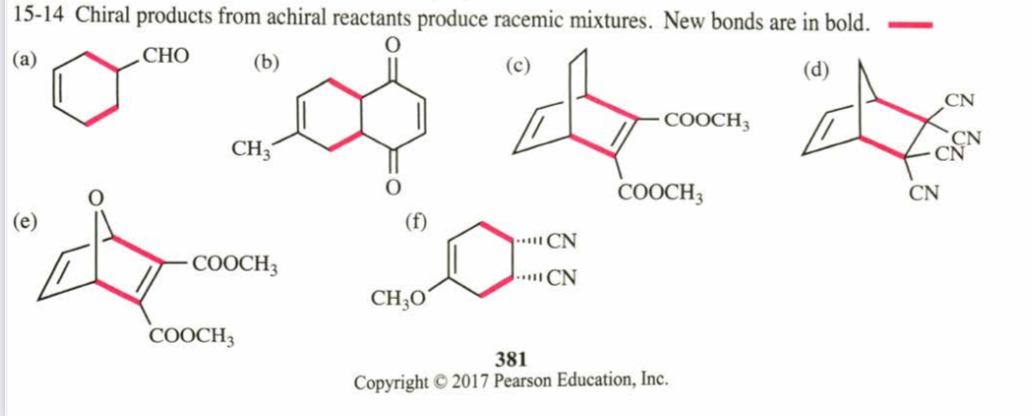
(15.14) Predict the products of the following proposed Diels–Alder reactions.
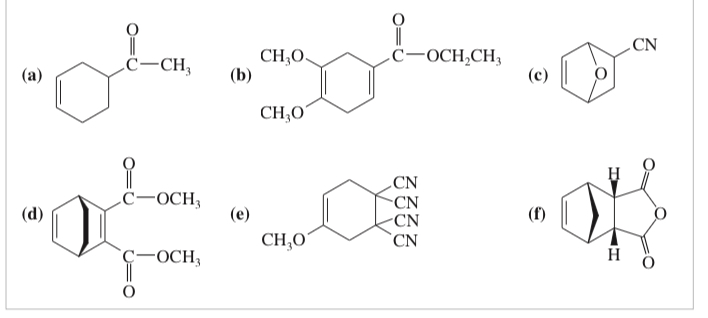
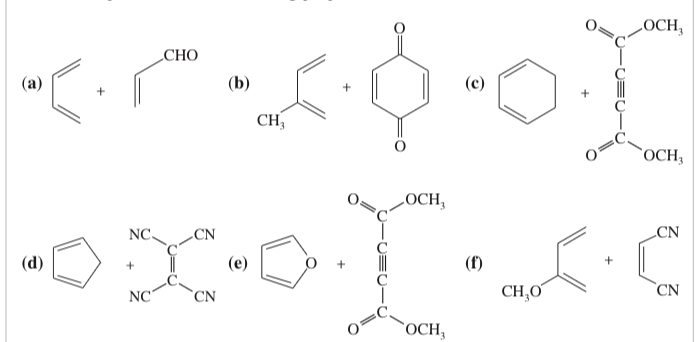
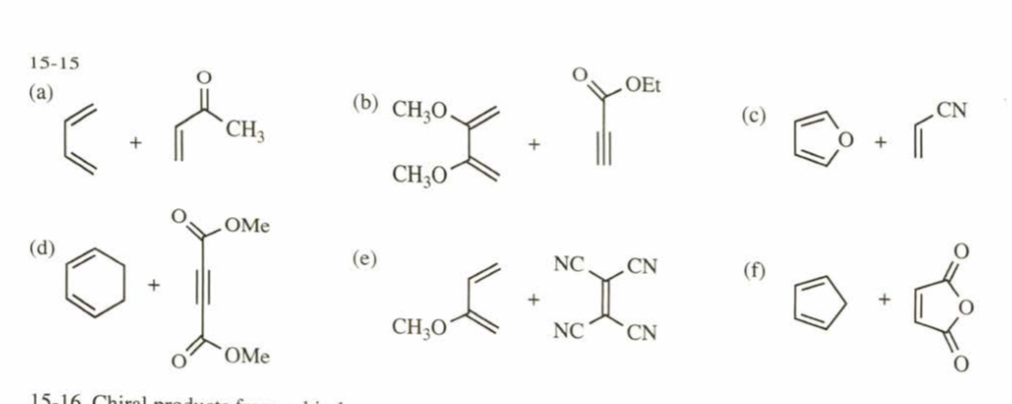
(15.15) What dienes and dienophiles would react to give the following Diels–Alder products?
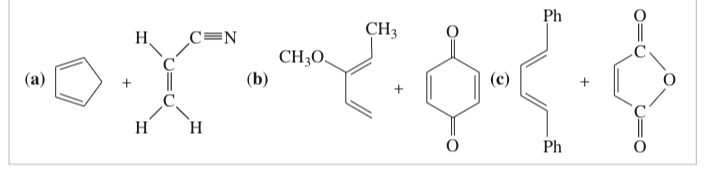
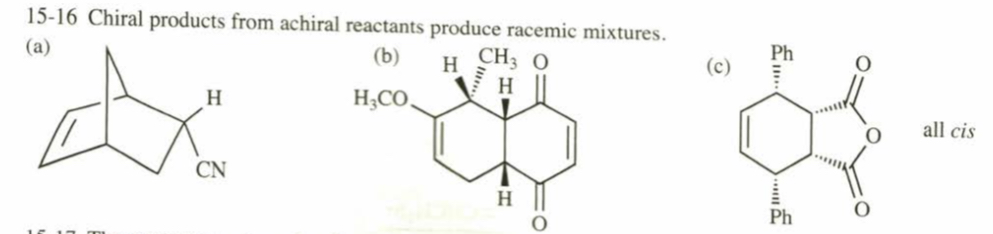
(15.16) Predict the major product for each proposed Diels–Alder reaction. Include stereochemistry where appropriate.
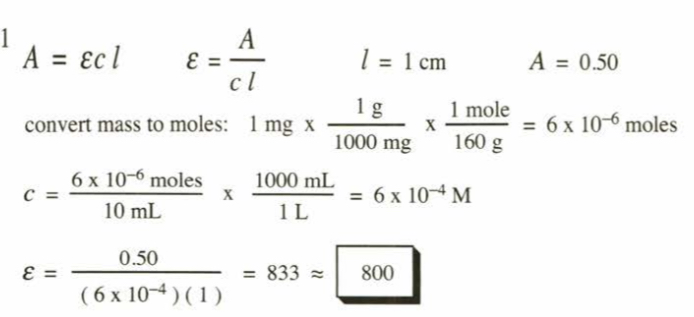
(15.21) One milligram of a compound of molecular weight 160 is dissolved in 10 mL of ethanol, and the solution is poured into a 1-cm UV cell. The UV spectrum is taken, and there is an absorption at lmax = 247 nm. The maximum absorbance at 247 nm is 0.50. Calculate the value of e for this absorption.
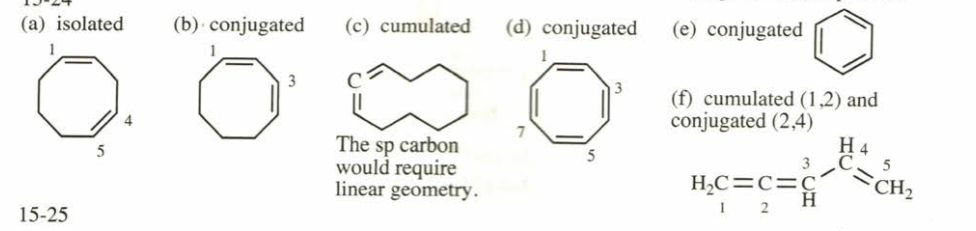
(15.24) Classify the following dienes and polyenes as isolated, conjugated, cumulated, or some combination of these classifications.
(a) cycloocta-1,4-diene (b) cycloocta-1,3-diene (c) cyclodeca-1,2-diene (d) cycloocta-1,3,5,7-tetraene (e) cyclohexa-1,3,5-triene (benzene) (f) penta-1,2,4-triene
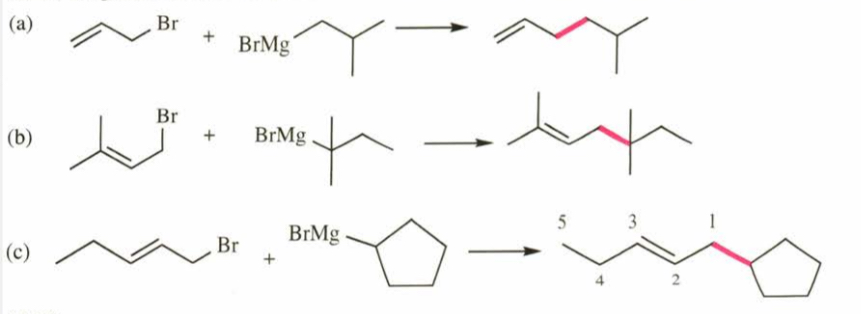
(15.26) Show how the reaction of an allylic halide with a Grignard reagent might be used to synthesize the following hydrocarbons. (a) 5-methylhex-1-ene (b) 2,5,5-trimethylhept-2-ene (c) 1-cyclopentylpent-2-ene
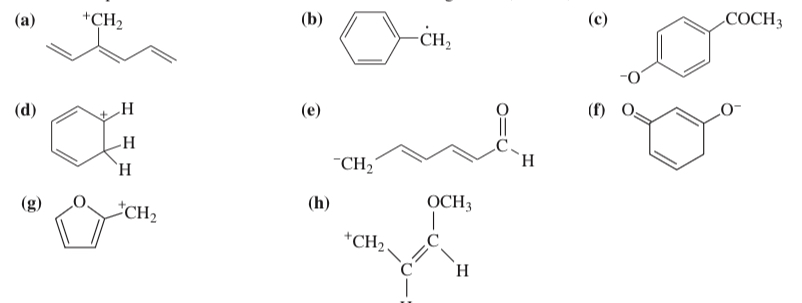
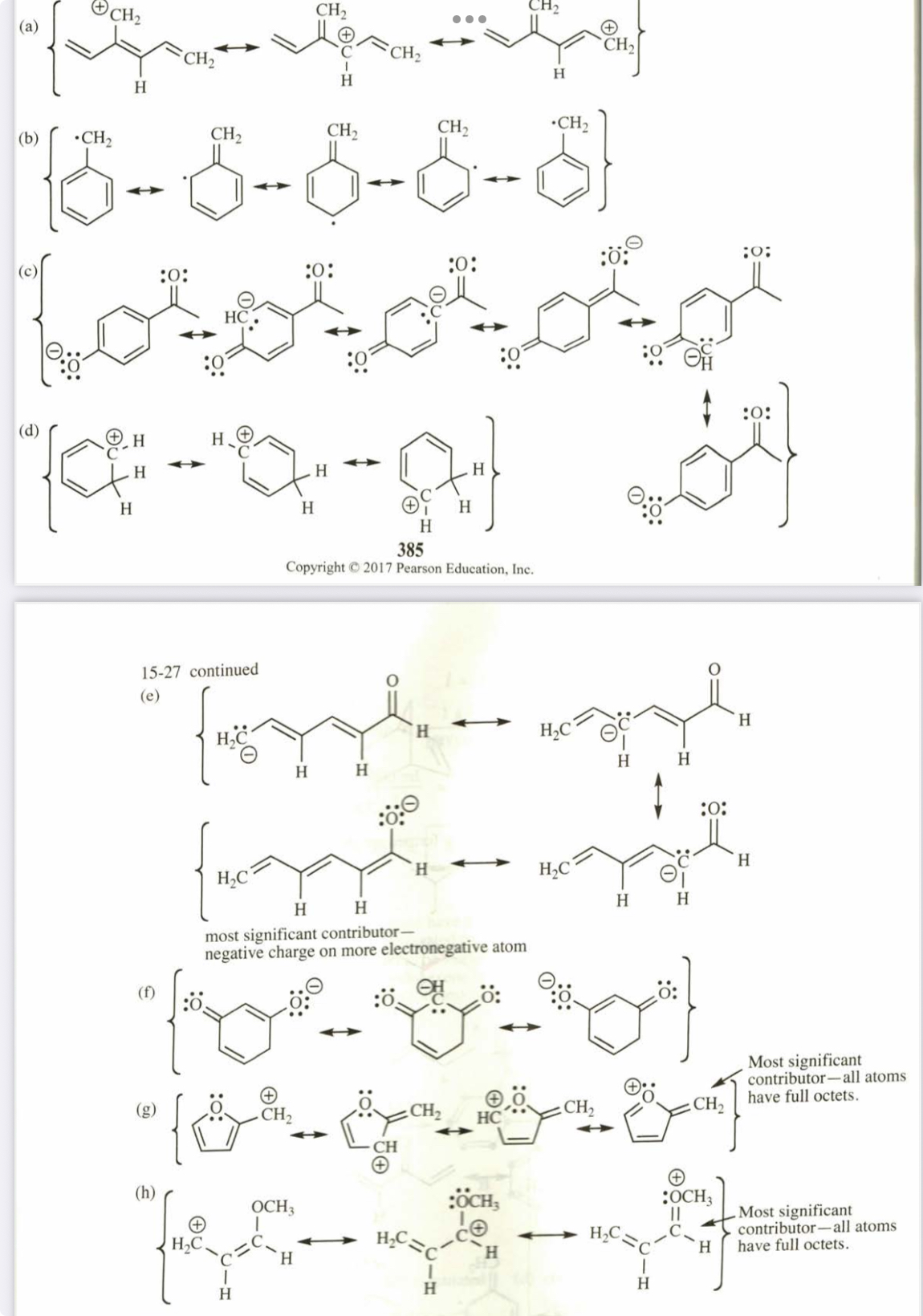
(15.27) Draw the important resonance contributors for the following cations, anions, and radicals
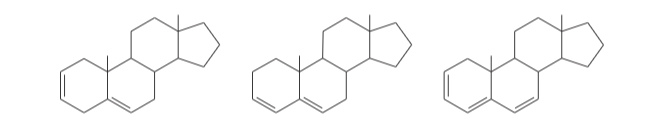
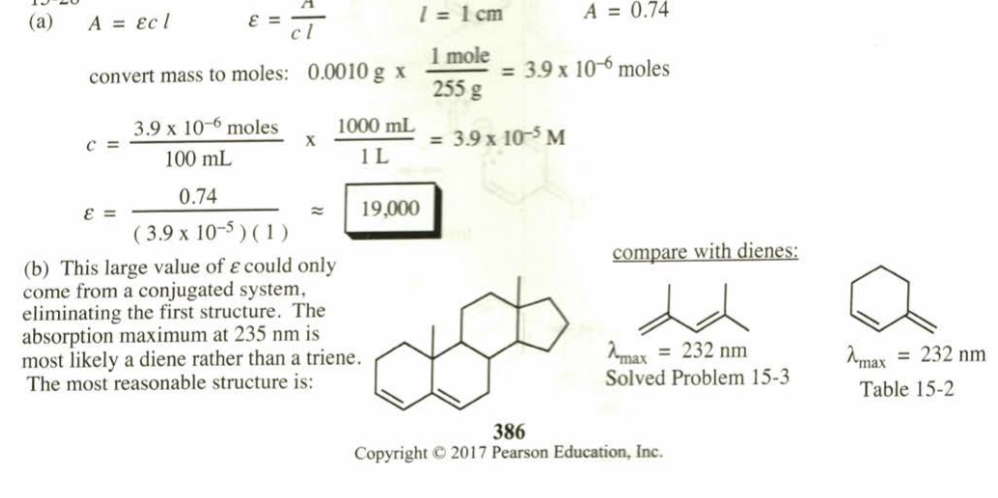
(15.28) A solution was prepared using 0.0010 g of an unknown steroid (of molecular weight around 255) in 100 mL of ethanol. Some of this solution was placed in a 1-cm cell, and the UV spectrum was measured. This solution was found to have lmax = 235 nm, with A = 0.74.
(a) Compute the value of the molar absorptivity at 235 nm.
(b) Which of the following compounds might give this spectrum?
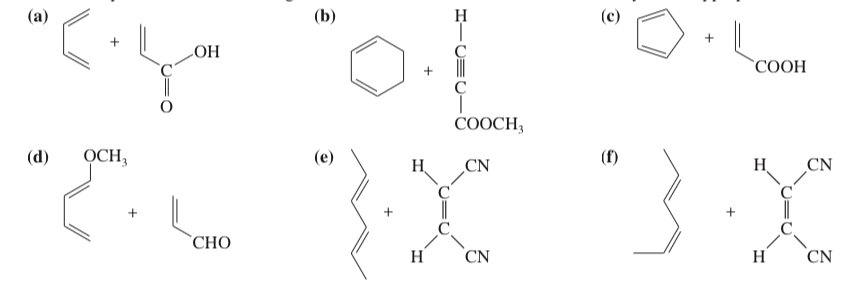
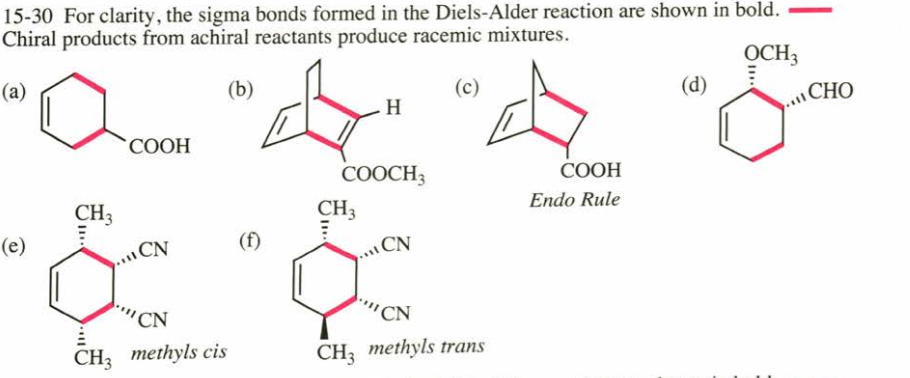
(15.30) Predict the products of the following Diels–Alder reactions. Include stereochemistry where appropriate.
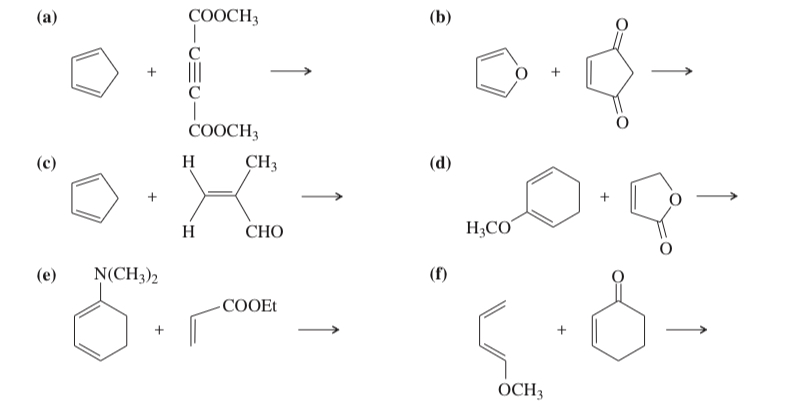
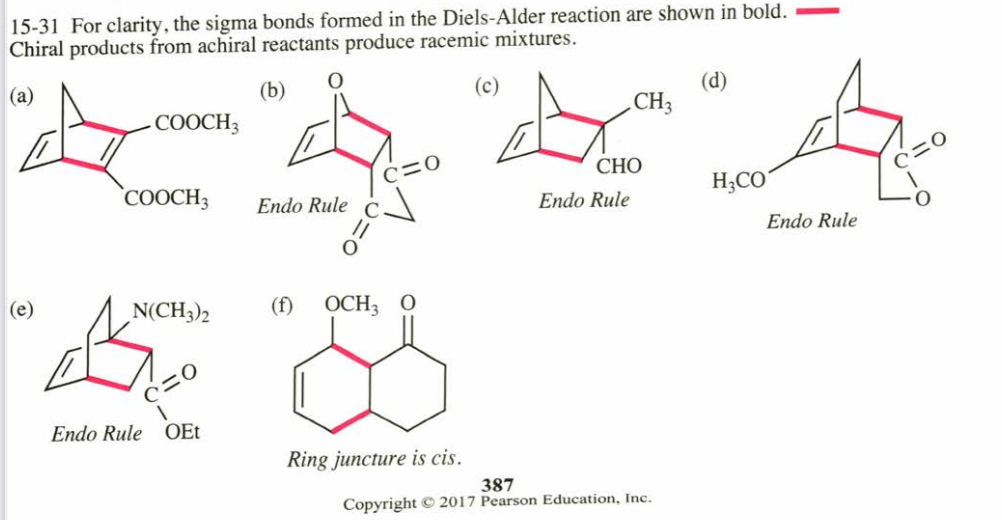
(15.31) Predict the products of the following reactions, including stereochemistry where applicable.
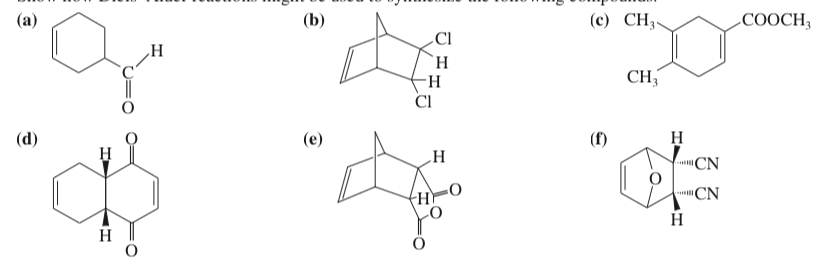
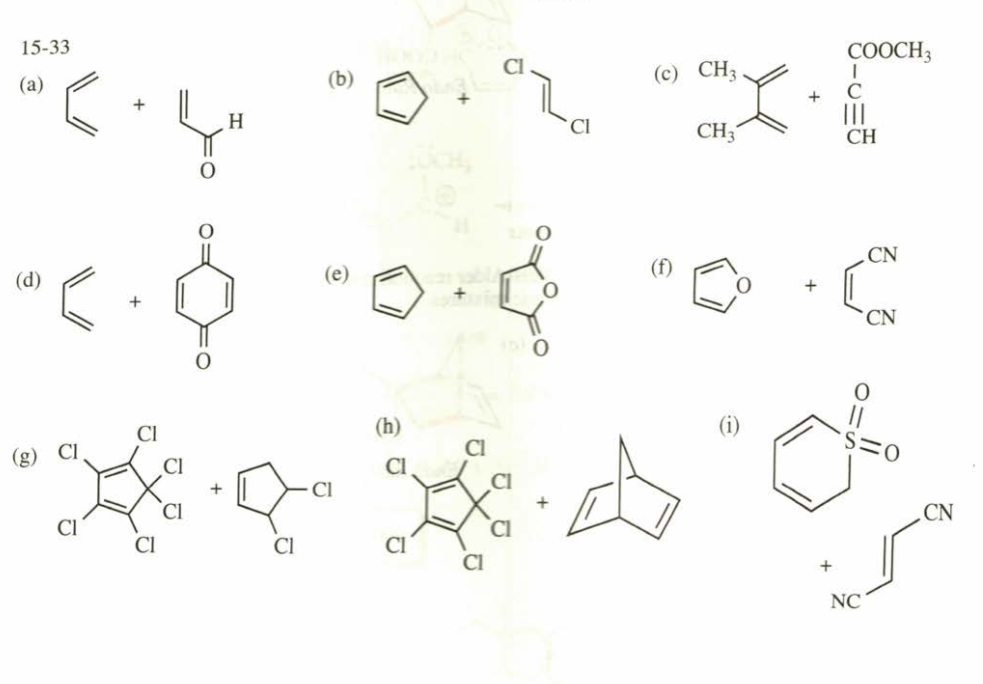
(15.33) Show how Diels–Alder reactions might be used to synthesize the following compounds.