Moles: Chemical reactions: Chemistry: (9:1)
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
Mole
Avogadro's number of an atom, ion or compound
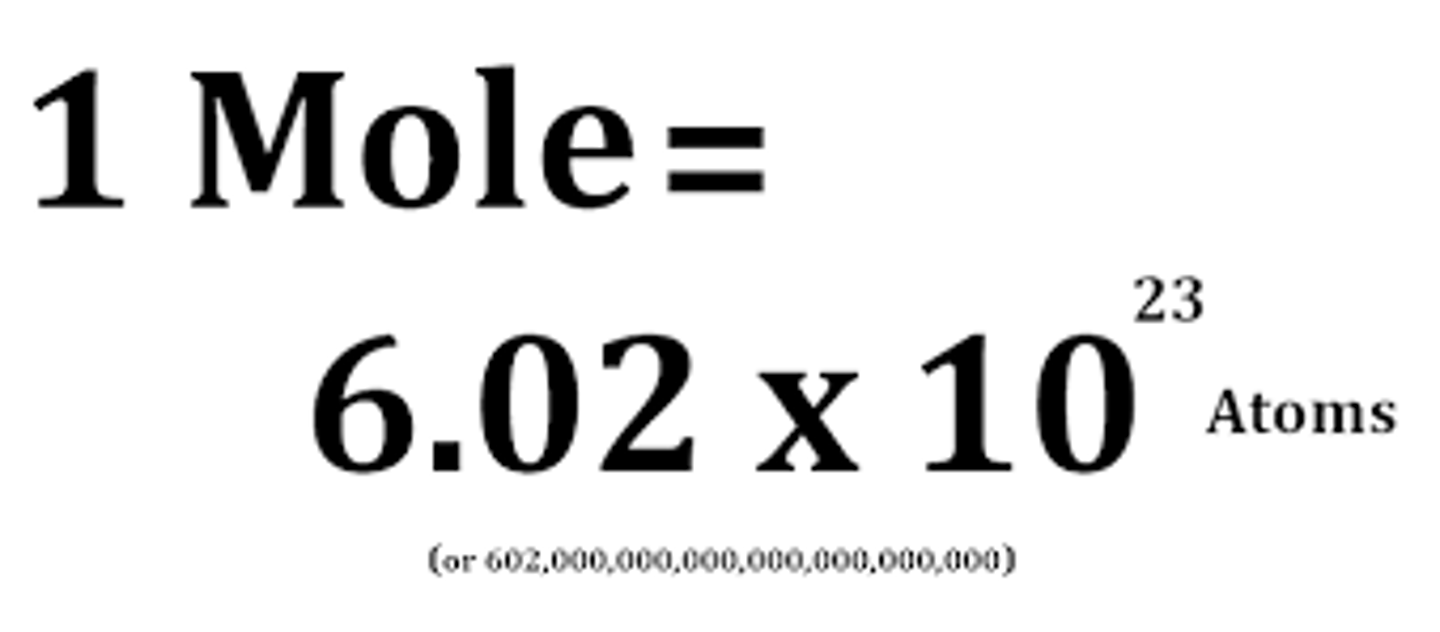
Formula for moles
Mass/relative formula mass (Mr)
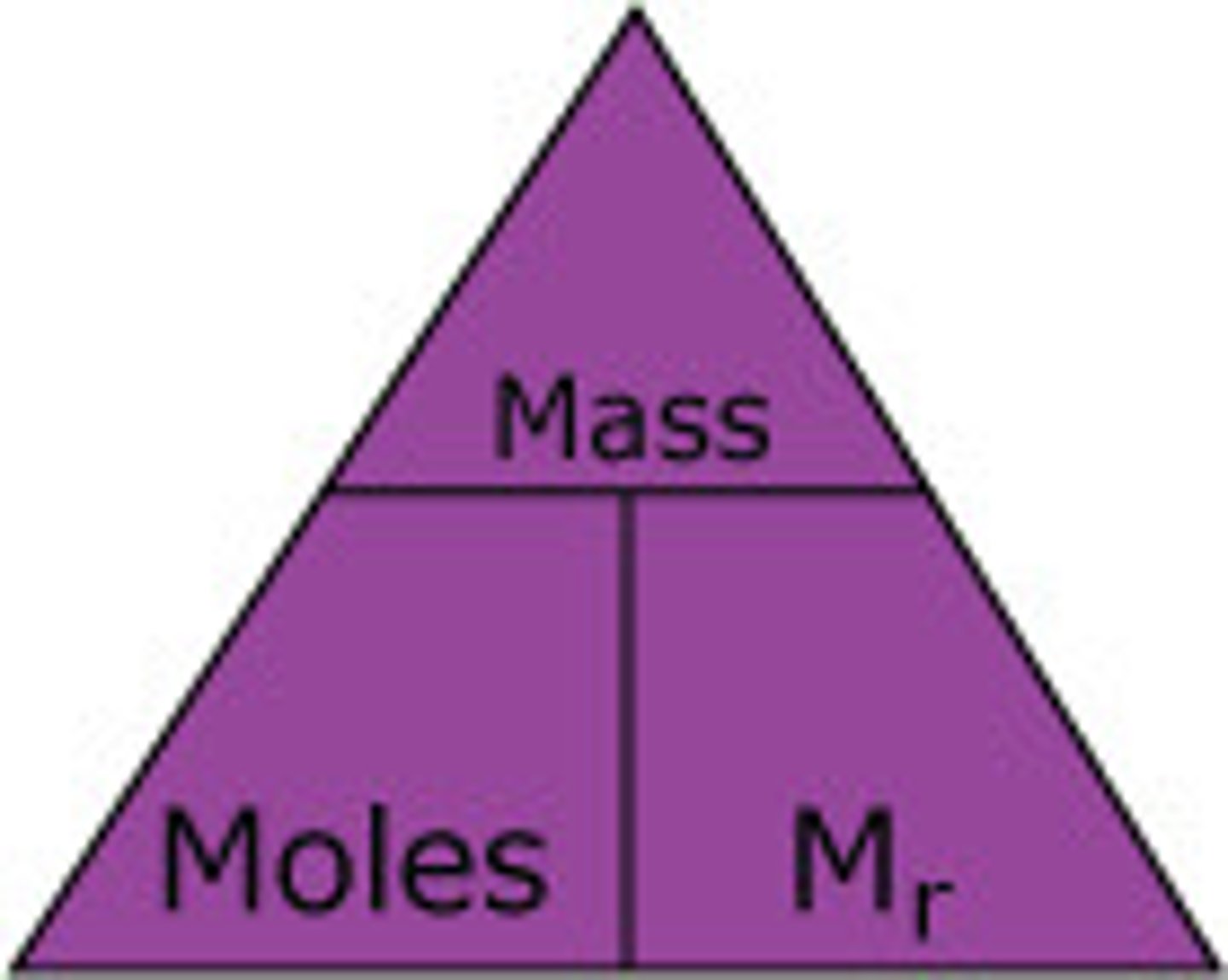
The mass of 1 mole of any substance=
formula mass in grams
mol
Symbol for moles
Value of Avogadro constant
6.02 x 10^23

Avogadro's constant
The number of atoms, molecules or ions in a mole of a given substance
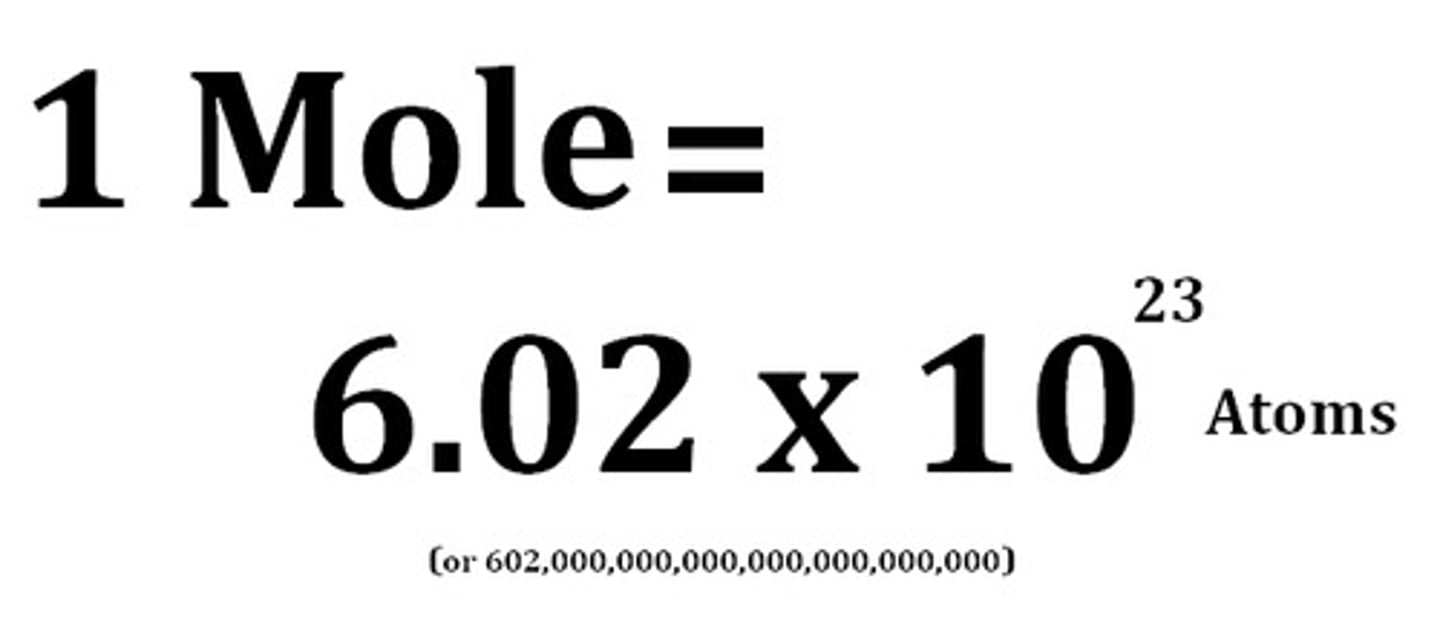
Relative atomic mass
The weighted mean mass of an atom of an element compared with one-twelfth of the mass of an atom of carbon-12.
Relative formula mass
The relative atomic masses of the elements in a compound added together
Reacting mass
The amount of one reactant needed to react exactly with another reactant.
Link between moles and equations
Multipliers show you the ratio of moles in a reaction
Reagent
Reactant
Limiting reactant (limiting reagent)
A reactant that is totally used up so it determines the amount of product formed.
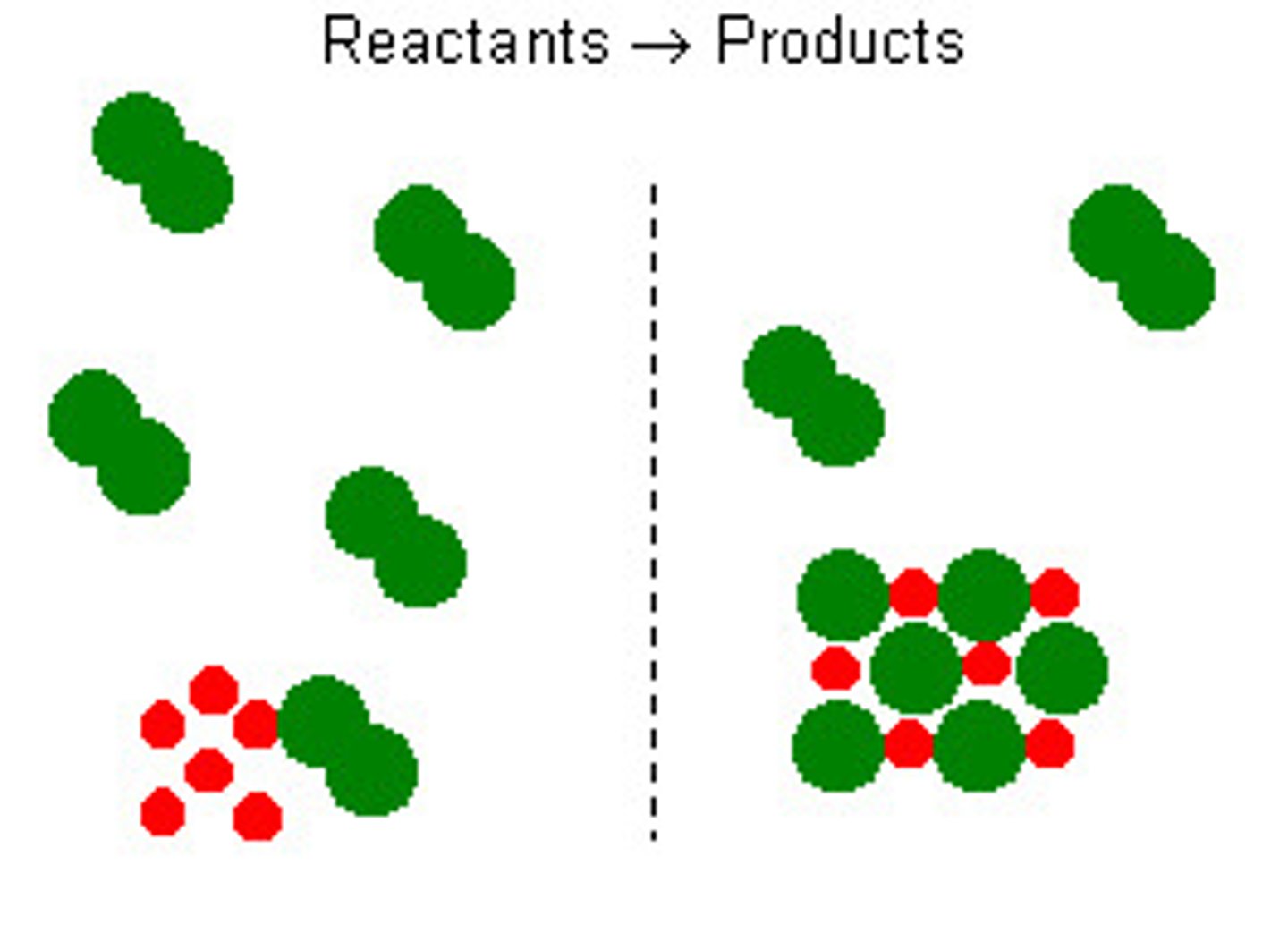
Excess
More than is required to react with the limiting reactant
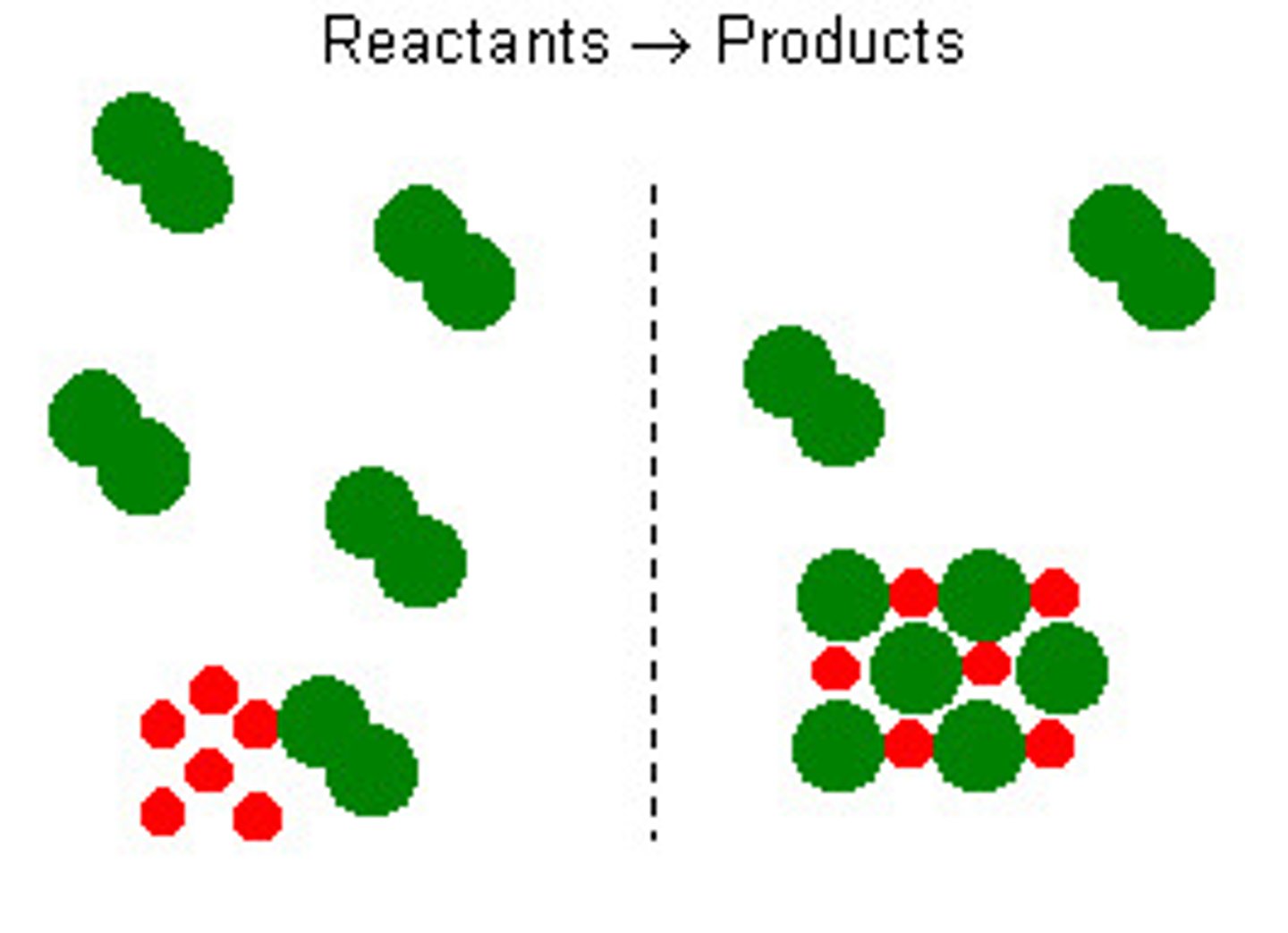
Stoichiometry of a reaction
The ratio of substances in a balanced equation