Learning Objectives: Chemistry
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
List the elements that make up the majority of living organisms
CHNOPS ( carbon A#6 mass-12 , hydrogenA#1 mass-1, nitrogen A#7 mass- 14, oxygen A#8 mass-16 , phosphorus, and sulfur)
Calculate molecular mass (mole. mass and weight are same)
C12H22O11. These show the number of each molecule involved. The atomic mass for these numbers are C=12, H=1, and O=16. Take how many molecules and multiply it by the atomic mass. This example = 342
Compare electronegativity values of different elements
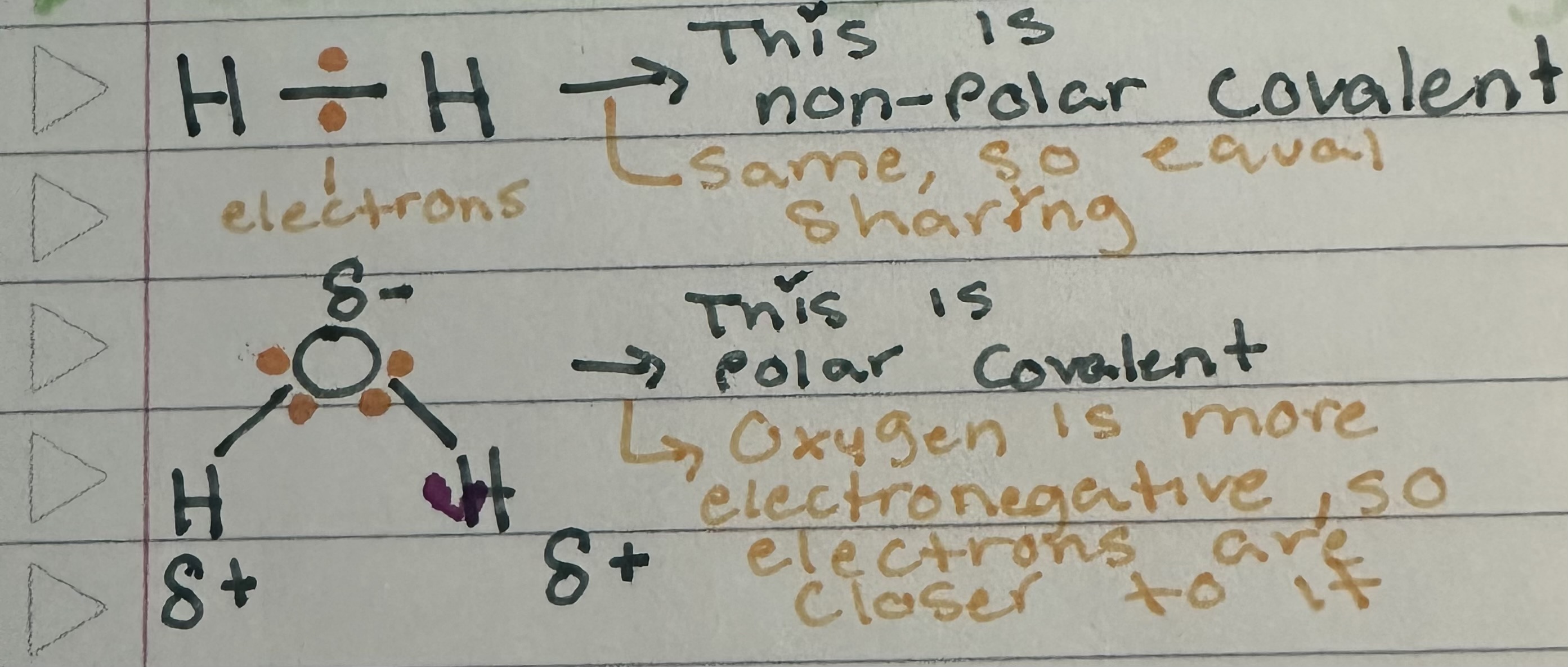
Define covalent bonds
The sharing of a pair of valence electrons by 2 atoms
Calculate Ph
--log10(H+)
Greater H+ concentration = lower pH - acidic
Lower H+ concentration = higher pH - basic
Calculate hydrogen ion concentration and molarity
[H+] = 10^-pH and converting pH to [H+] by taking 10 to the power of the negative pH and converting the moles of solute and volume in liters for molarity, or using the inverse relationship to find moles from molarity and volume.
List the features of water molecules that result in its label as universal solvent
It is polar, has hydrogen bonding, can dissolve ionic compounds
what are the functional groups
Hydroxyl, carbonyl, carboxyl, amino, sulfhydryl, phosphate, and methyl groups
Define polar covalent bonds
One atom is more electronegative (NOT evenly shared) - this causes a partial pos/neg charge for each molecule
Define non polar covalent bonds
Atoms share the electron equally (evenly shared)
Define ionic bonds
An attraction between an anion and a cation (transfer of electrons)
Define hydrogen bonds
A weak attraction that forms between a hydrogen atom covalently bonded to one highly electronegative atom is also attracted to ANOTHER electronegative atom
Define Van Der Waals Forces
weaker than chemical bonds, but they are responsible for holding molecules together in liquids and solids and play a role in phenomena like surface tension ?
Define Cation
pawsitively charged ion
Define Anion
negatively charged ion
Compounds formed by ionic bonds are also called…
salts