ALL - THE PERIODIC TABLE + BONDING
1/109
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
110 Terms
How is the periodic table organised?
In order of atomic mass
Columns (groups) consist of…
Elements with similar chemical properties
Group = number of
Electrons in outer shell
Period = number of
Shells
What is group 1 called?
Alkali metals
What are group 7 called?
Halogens
What are group 0 called?
Nobel gases
Properties change as you go along…
Periods
Elemnts in the same group have the same number of electrons in their outer shell, what does this mean they also have?
Similar properties.
What do the properties of elements depend on?
The number of electrons they have
What is the atomic number equal to?
Number of electrons (proton number)
How do you work out electron configuration through period and group?
Number of shells = period
Number of outer shell electrons = group number
Where are the metals and non-metals on the periodic table?
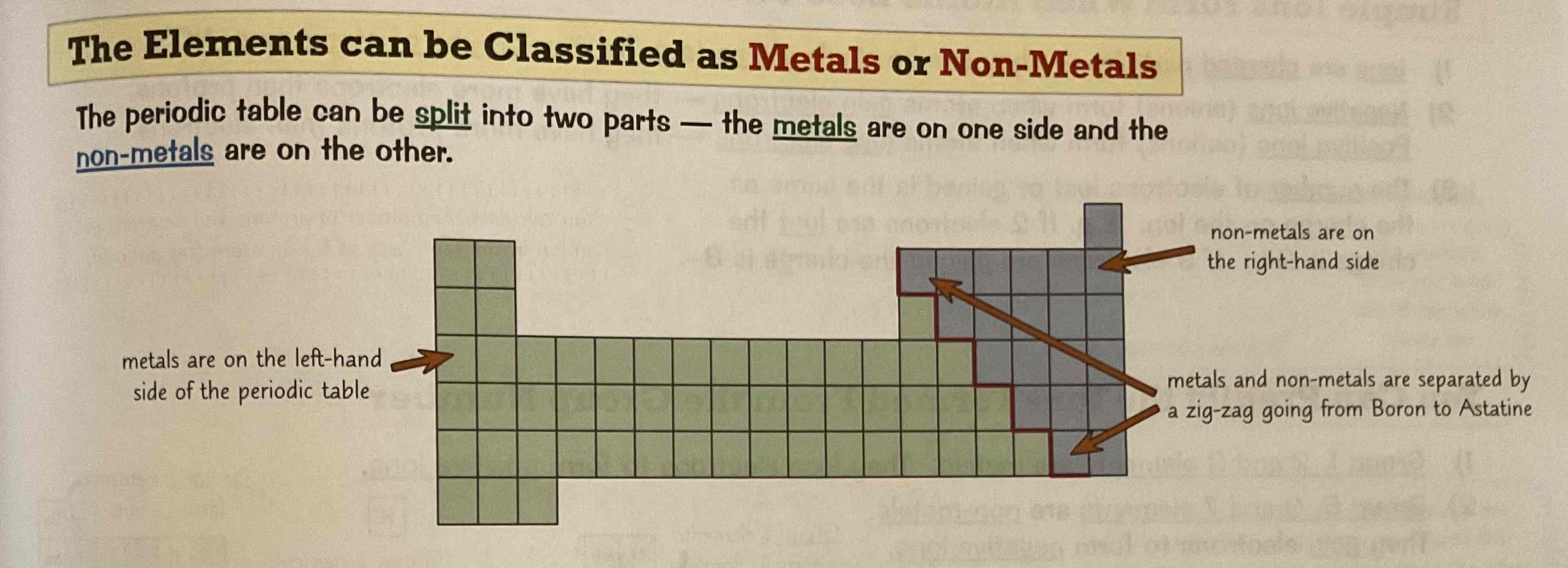
Do metals conduct electricity? Why?
Metals DO conduct electricity because they allow charge to pass through them easily
Are metal oxides basic or acidic? What does this mean?
Metal oxides are BASIC. This means they will neutralise acids.
What will the pH of a metal oxide which dissolves be?
More than 7
Are non-metals good conductors of electricity?
Poor conductors of electricity
Are non-metal oxides basic or acidic? What does this mean?
Acidic - they will neutralise bases
When non-metal oxides dissolve in water what pH do they form?
Less than 7
How can all group 7 elements be described? (Physical)
Inert, colourless gases
What are group 0 elements called?
Nobel gases
What are some examples of Nobel gases?
Helium, neon, argon
Do Nobel gases react? (Technical term)
No not much at all, they are INERT
Why are Nobel gases inert?
It takes a lot of energy to add or remove electrons from a Nobel gas atom
Do Nobel gases exist in pairs or as single atoms? Why?
No, they exist as single atoms, because they are inert
What are ions?
Charged particles (can be single atoms (e.g. Na+) or groups of atoms (e.g.NO3-))
What are anions?
Negative ions
How do anions form?
When atoms GAIN electrons - more electrons than protons
What are cations?
Positive ions
How do cations form?
When atoms LOSE electrons - more protons than electrons
What is the charge equal to?
The number of electrons lost or gained. E.g. 2 electrons lost, charge = 2+
How can you predict the ions formed?
Group numbers
What ions do groups 1,2 and 3 form (metals)
lose electrons → positive ions
What ions do groups 5, 6 and 7 elements form? (Non-metals)
Gain electrons → negative ions
What ion does silver form? (Ag)
Ag+
What ion does iron (II) form (Fe)
Fe2+
What ion does copper form? (Cu)
Cu2+
What ion does Iron (III) form? (Fe)
Fe3+
What ion does lead form? (Pb)
Pb2+
What ion does zinc form (Zn)
Zn2+
What ion does hydrogen form? (H)
H+
What ion does hydroxide form? (OH)
OH-
What ion does ammonium form? (NH4)
N(H4)+
What ion does carbonate form? (CO3)
C(O3)2-
What ion does nitrate form? (NO3)
N(O3)-
What ion does sulfate form? (SO4)
S(O4) 2-
How are ionic compounds produced?
Transfer of electrons
What happens when a metal and non-metal react together?
Metal atom loses electrons → positive ion (cation), non-metal gains these electrons to form positive ion (anion)
What is the bond between oppositely charge ions?
They are strongly attracted to one another by electrostatic attractions
What is the attraction between oppositely charged ions called?
Ionic bond
What is the reaction between sodium and chlorine a classic case of?
Ionic bonding
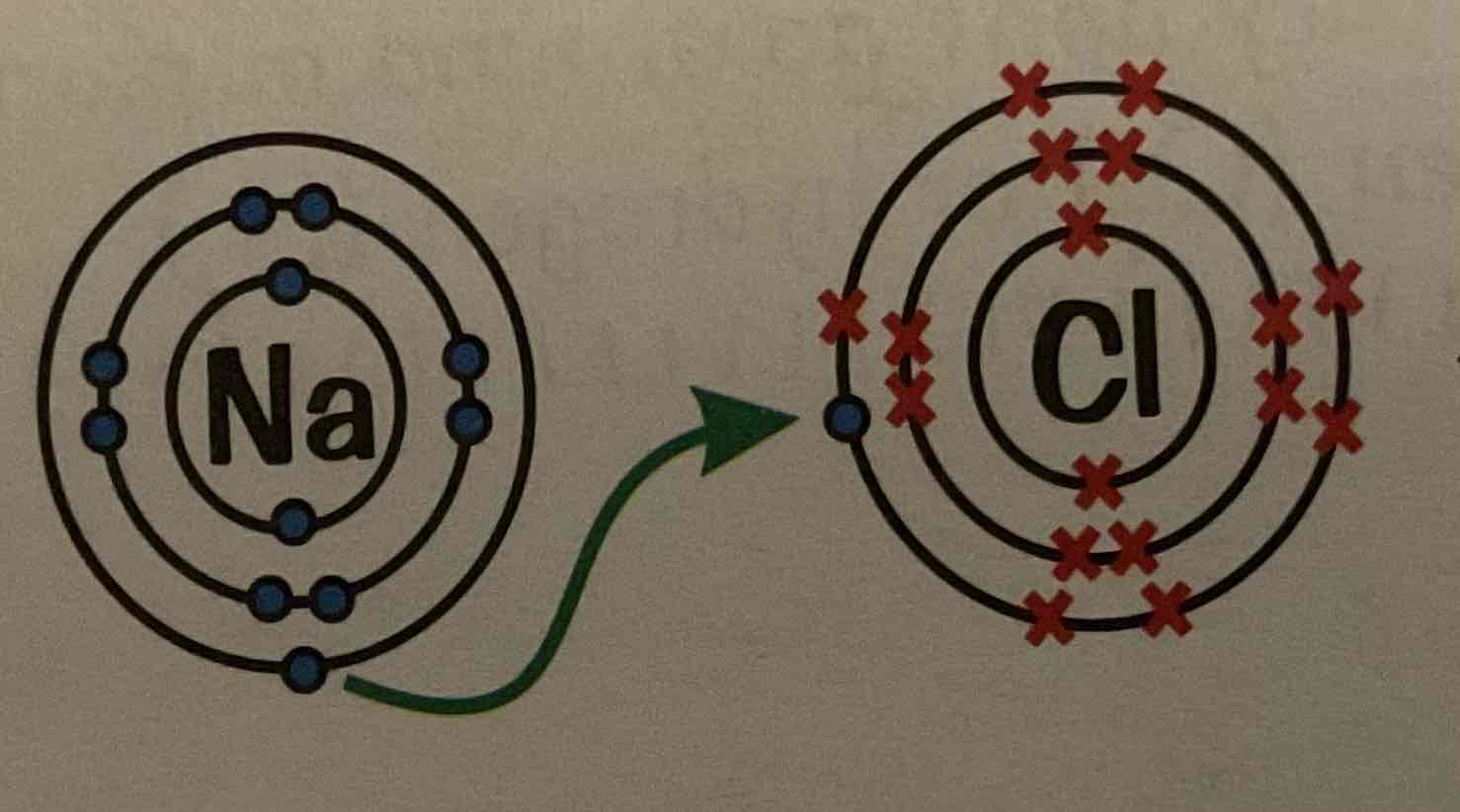
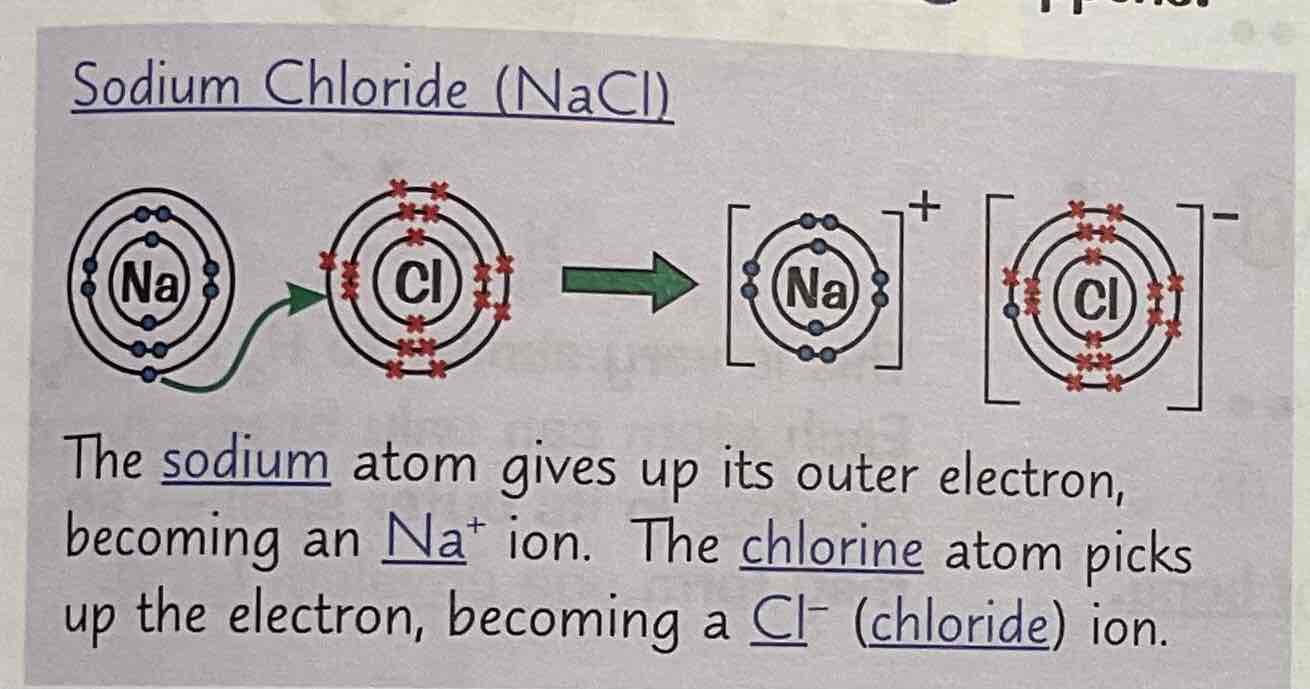
What happens when Sodium and chlorine react (ionic bonding)?
Sodium atom gives up its outer electron and becomes and Na+ ion.
The Chlorie atom picks up the spare electron and becomes a Cl- ion.
What are ionic compounds made up of?
A positively charged part and a negatively charged part
What can you use to work out the formula for the ionic compound?
The charges on the individual ions present
What diagrams can be used to show what happens to the electrons when ionic bonding happens?
‘Dot and cross’
Draw an example of sodium chloride - dot and cross (NaCl)
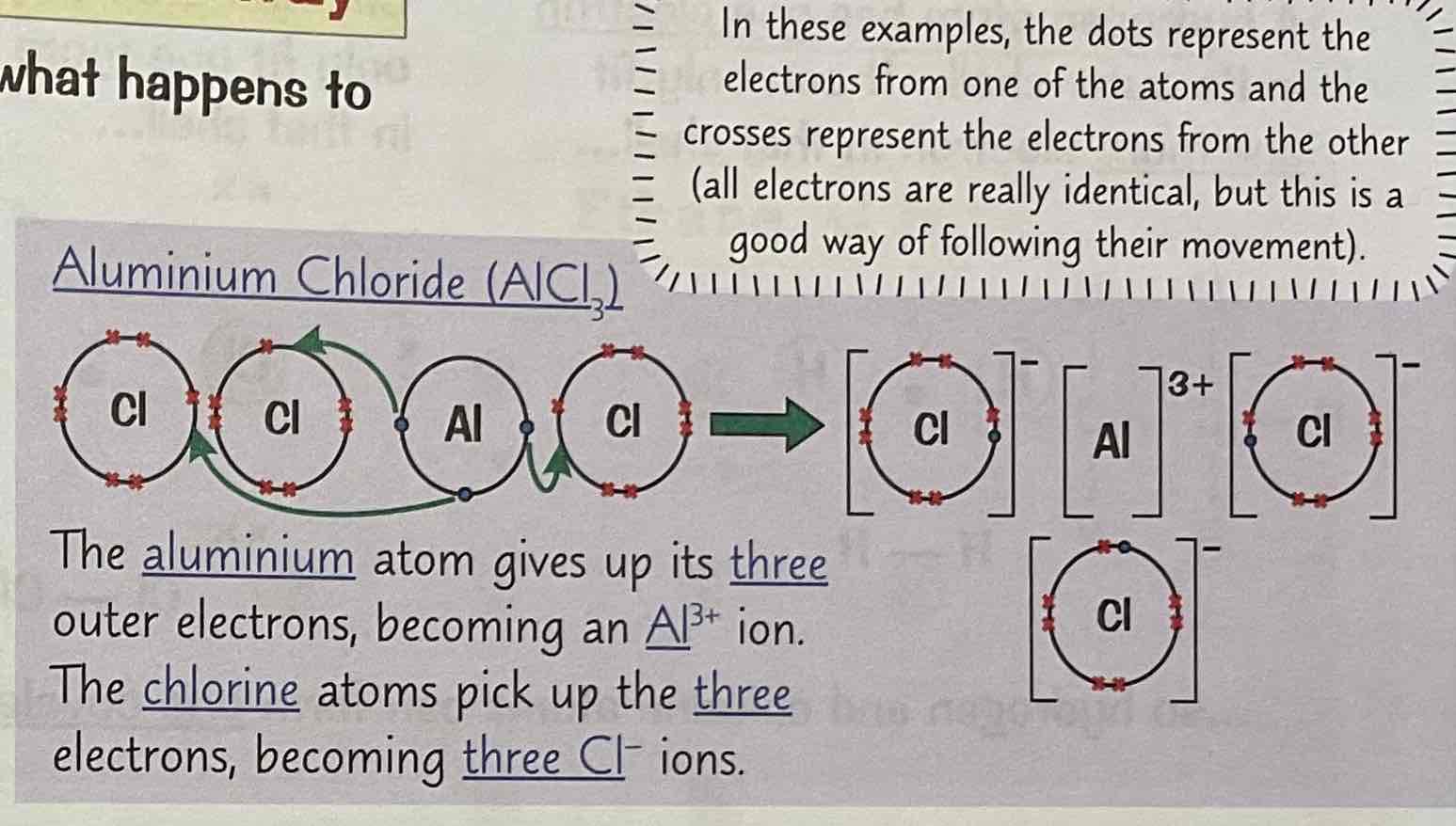
Draw a dot and cross diagram of Aluminium chloride (AlCl3)
Draw a dot and cross diagram of Magnesium Oxide (MgO)
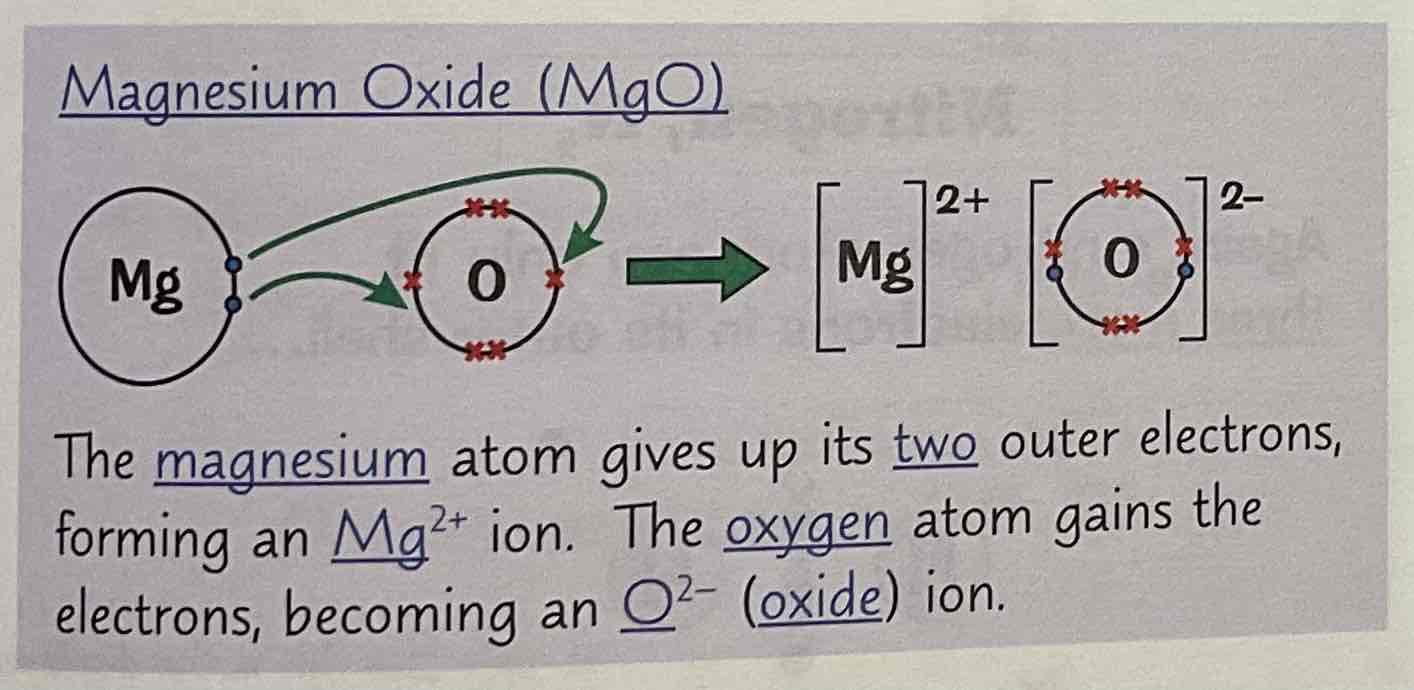
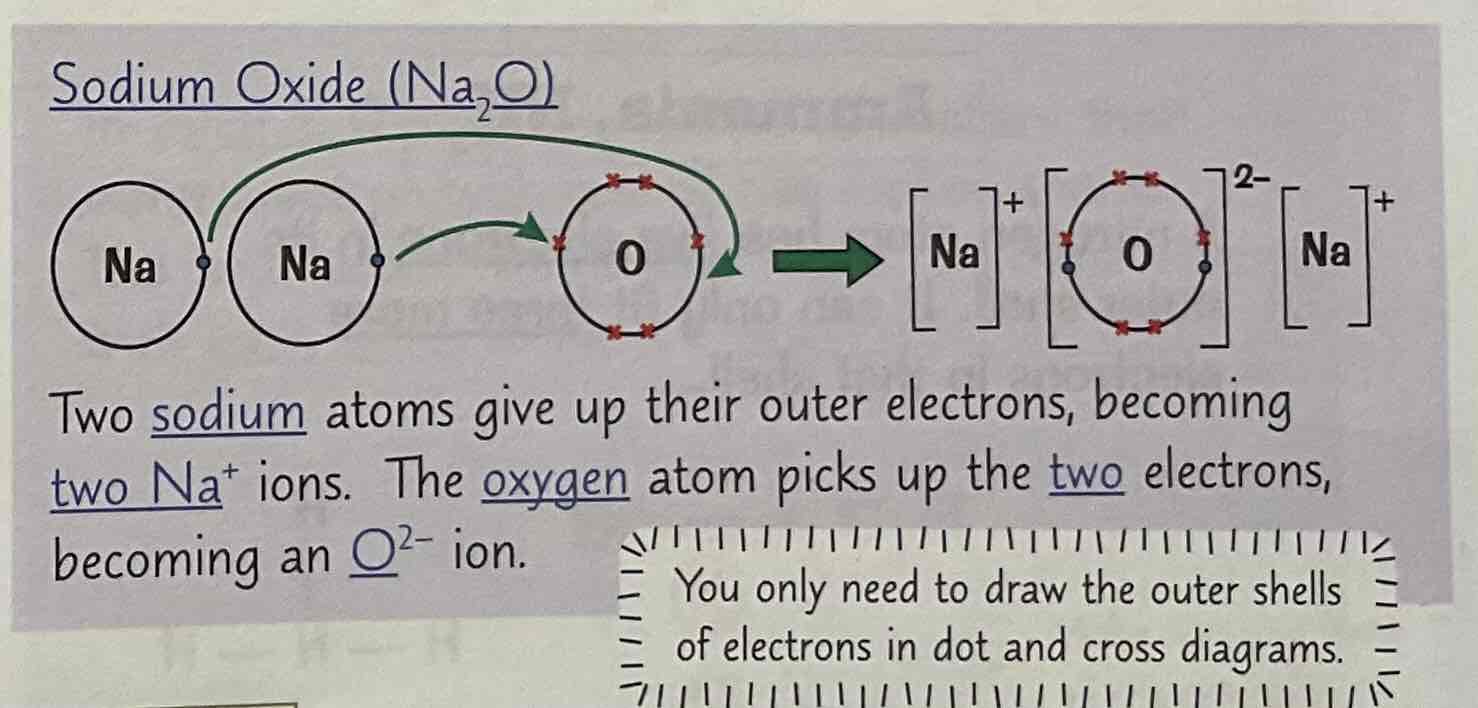
Draw a dot and cross diagram of Sodium Oxide (Na2O)
What sort of structure do ionic compounds have?
Lattice structure
What structure do compounds with ionic bonding always have?
Giant ionic structure
How are the ions held together?
By closely packed in a 3D lattice arrangement by the attraction between oppositely charged ions
Do ionic compounds have high or low melting and boiling points?
High
Why are ionic compounds melting and boiling points high/low?
High - the electrostatic attraction between oppositely charged ions is very strong. Because a lot of energy is needed to overcome the strong attraction
Do ionic compounds conduct electricity when solid?
No
When can ionic compounds conduct electricity?
Molten or dissolved in water
What do covalent substances contain?
Shared pairs of electrons
How do atoms make covalent bonds?
Sharing pairs of electrons with other atoms
What does each covalent bond provide?
One extra shared electron for each atom
What is the attraction like in covalent bonding?
Strong electrostatic attraction between the negatively charged shared electrons (the bonding pair) and the positively charged nuclei of the atoms involved
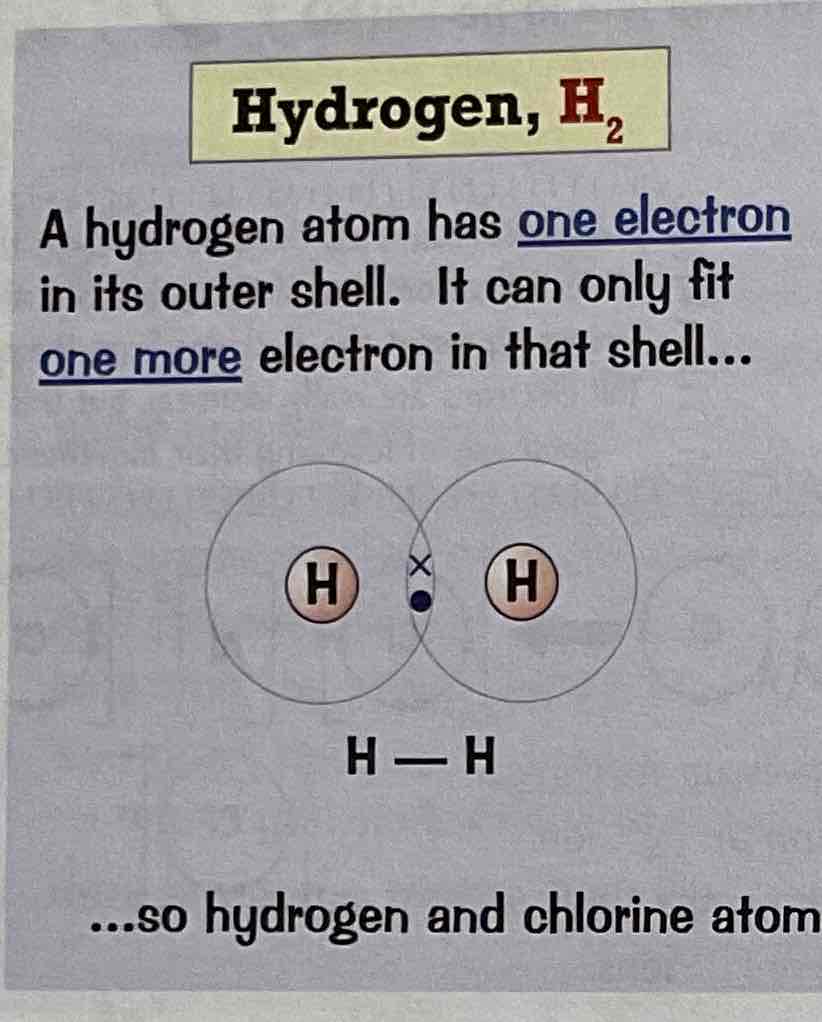
Draw a dot and cross diagram for the covalent bonding of H2
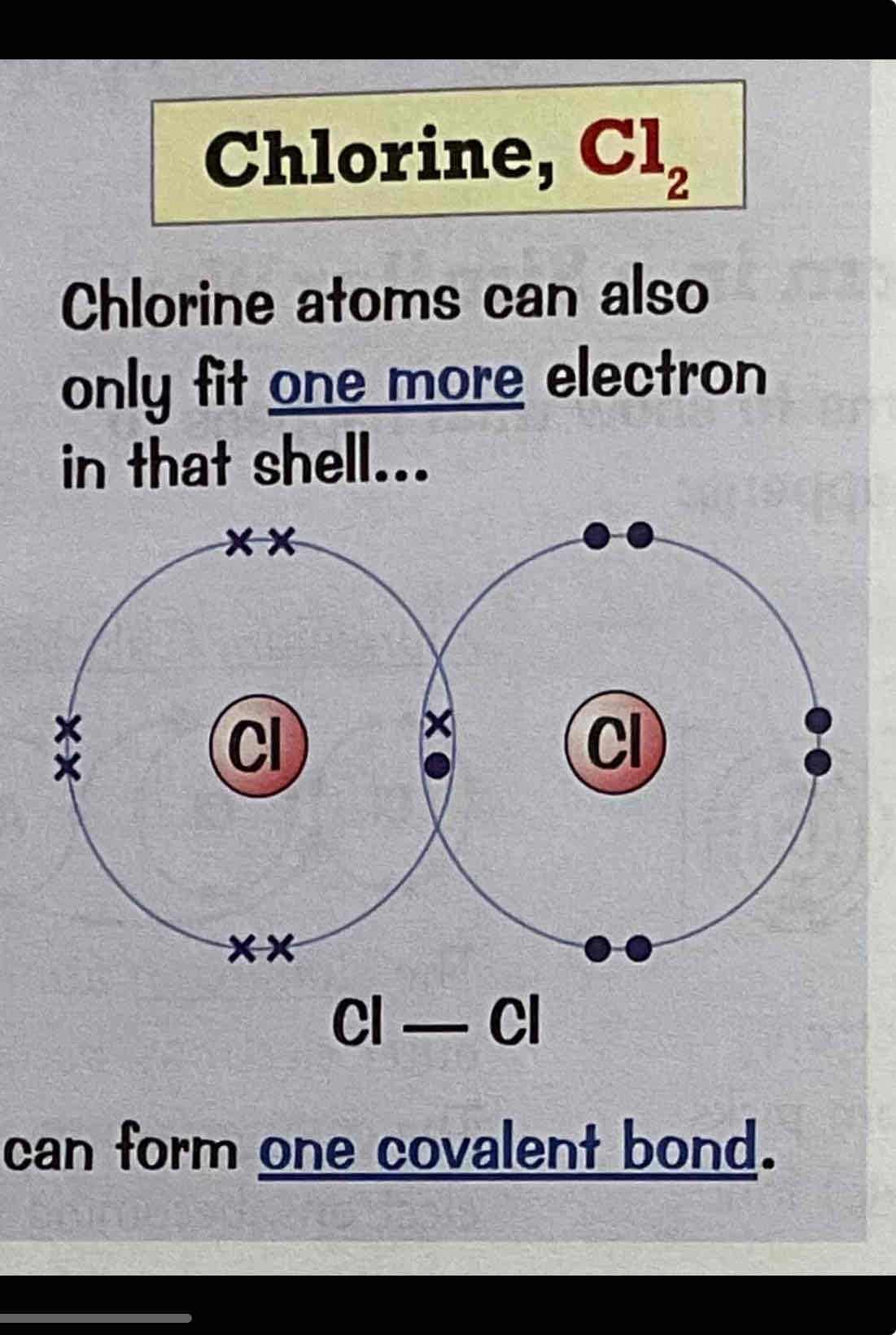
Draw a dot and cross diagram for the covalent boding of Chlorine, Cl2
Draw a dot and cross diagram for the covalent bonding of hydrogen chloride, HCl
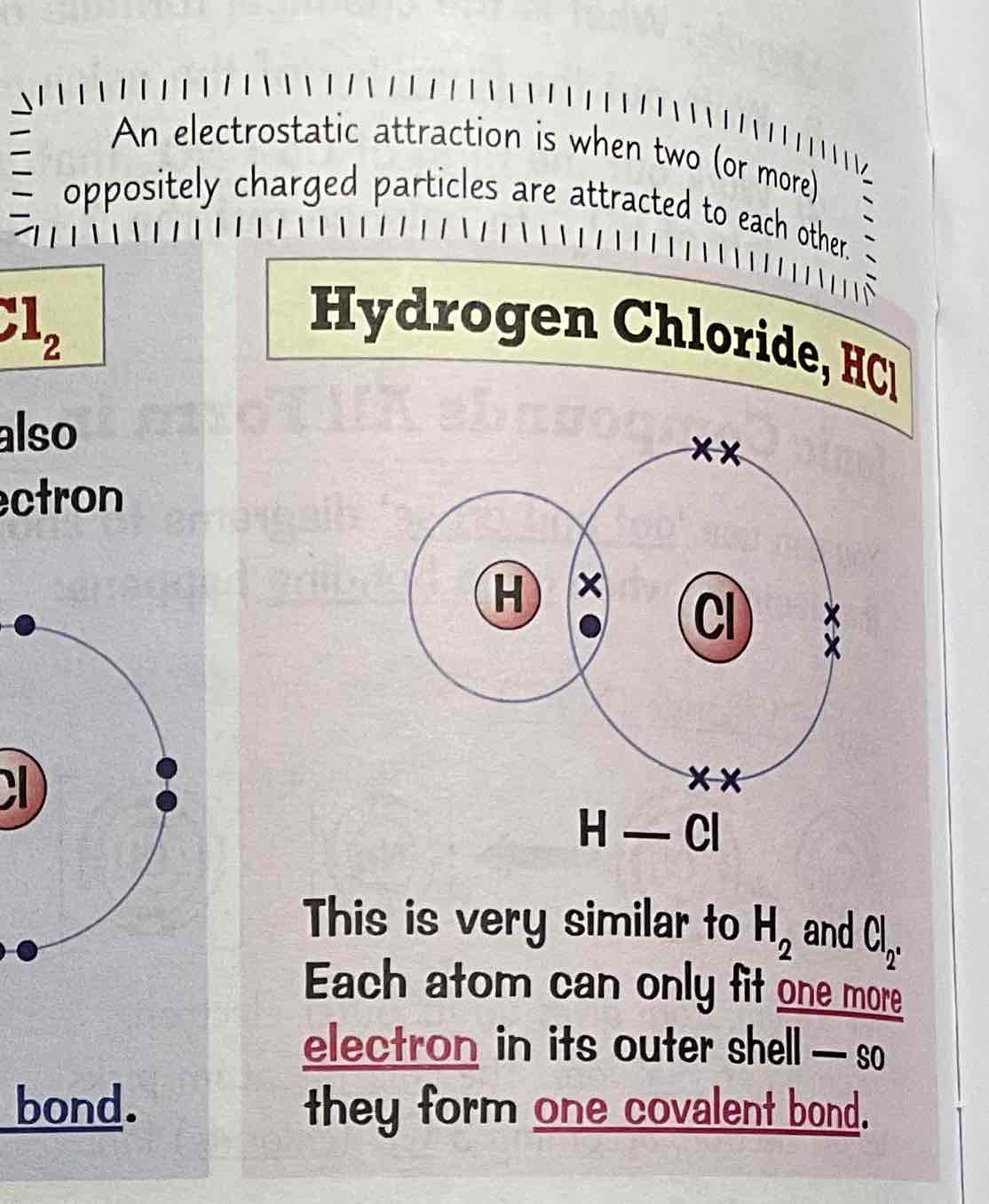
Draw dot and cross diagram for the covalent bonding of ammonia, NH3
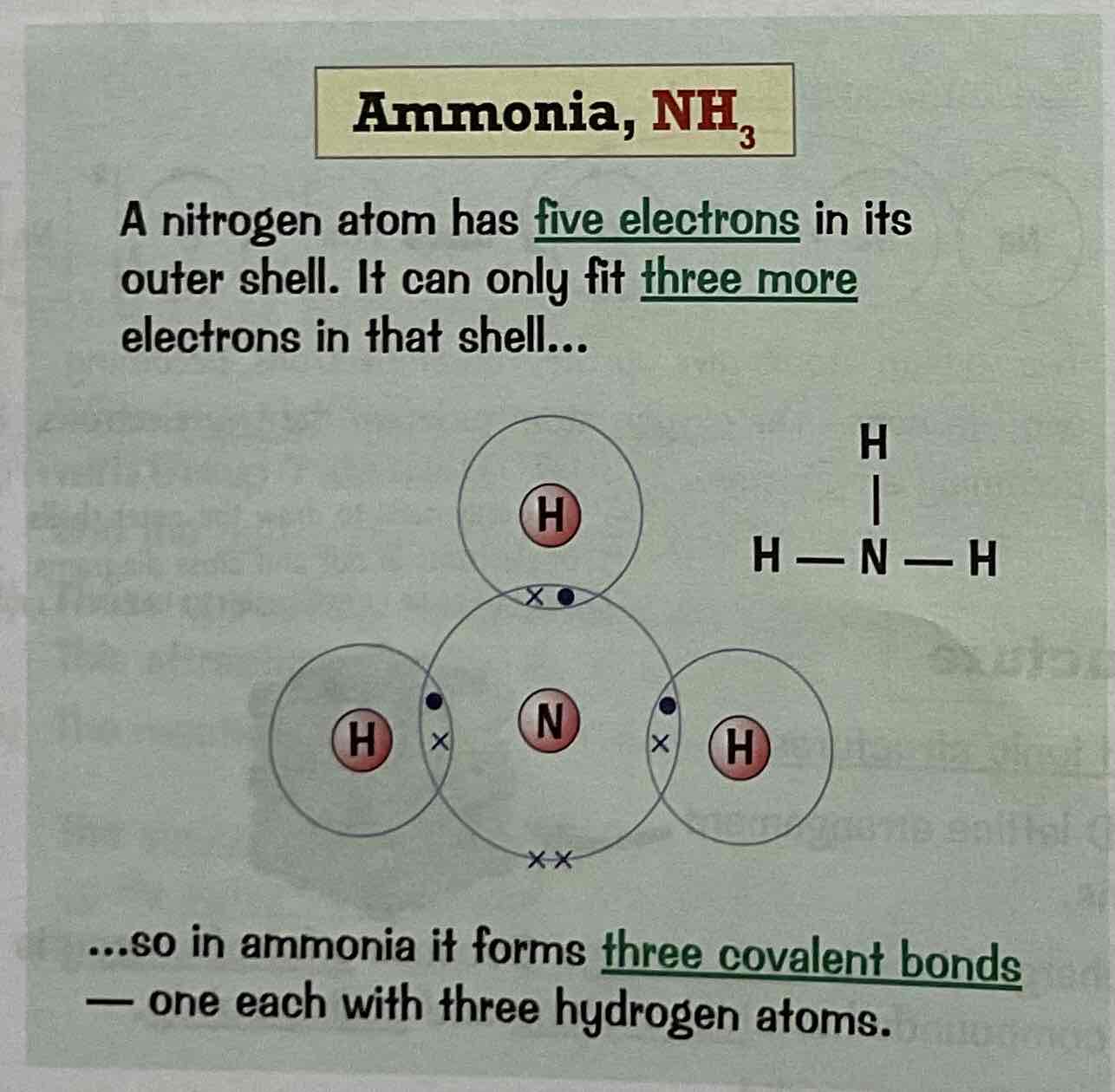
Draw a dot and cross diagram for the covalent bonding of Nitrogen, N2
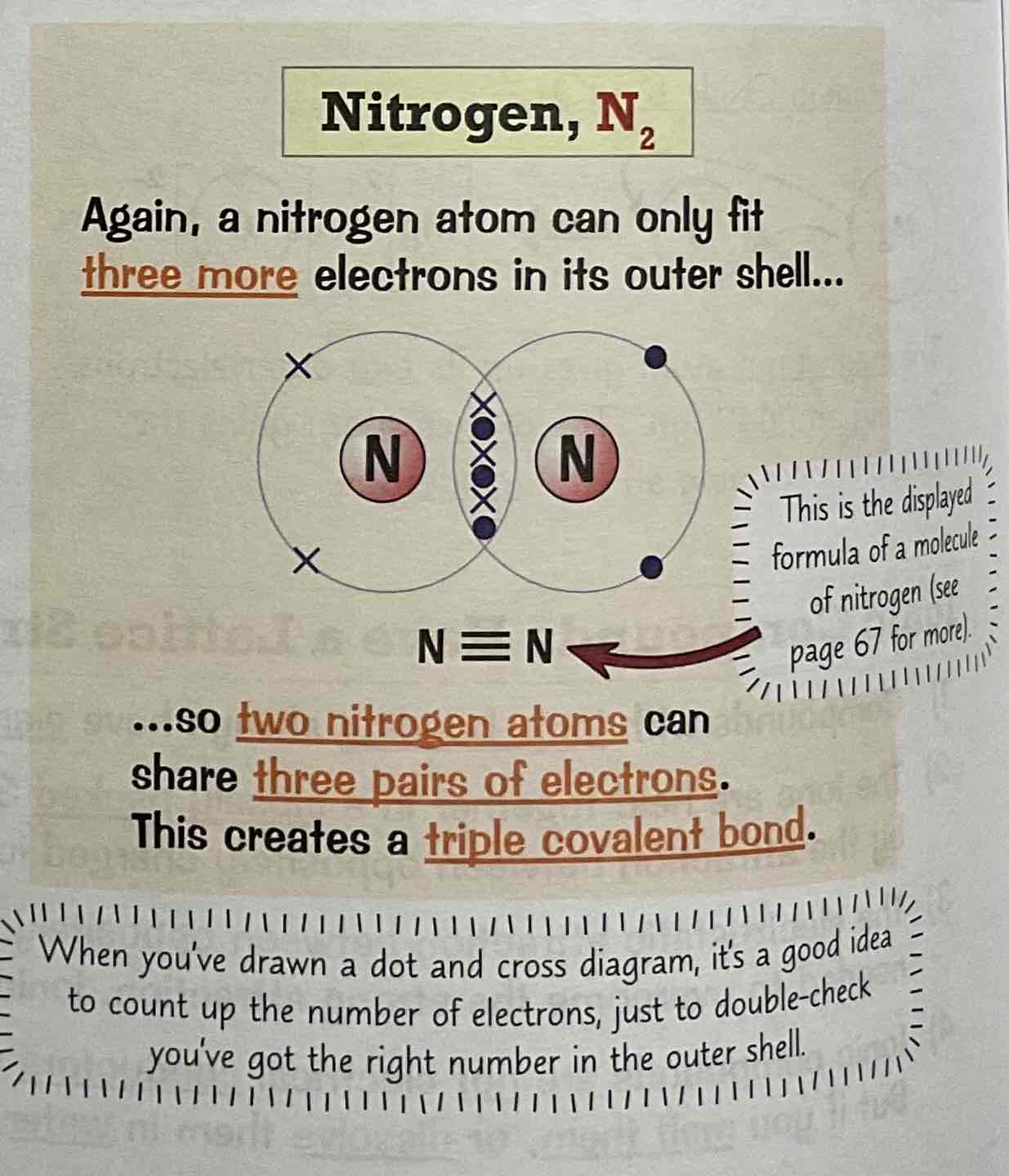
Draw a dot and cross diagram for the covalent bonding of Water, H2O
Draw a dot and cross diagram for the covalent bonding of oxygen, O2
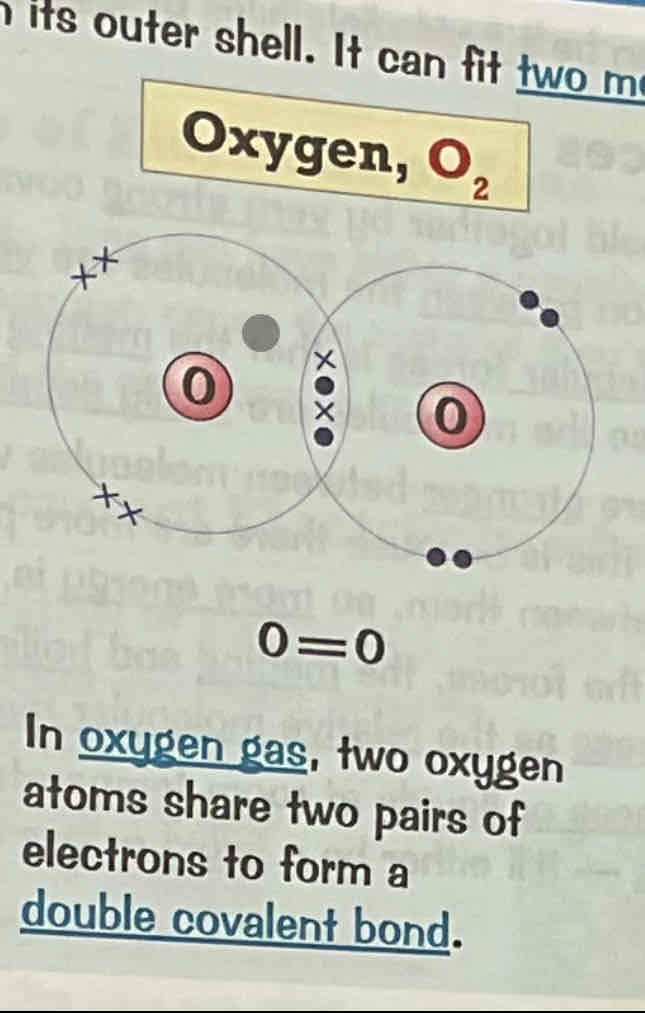
Draw a dot and cross diagram for the covalent bonding of carbon dioxide, CO2
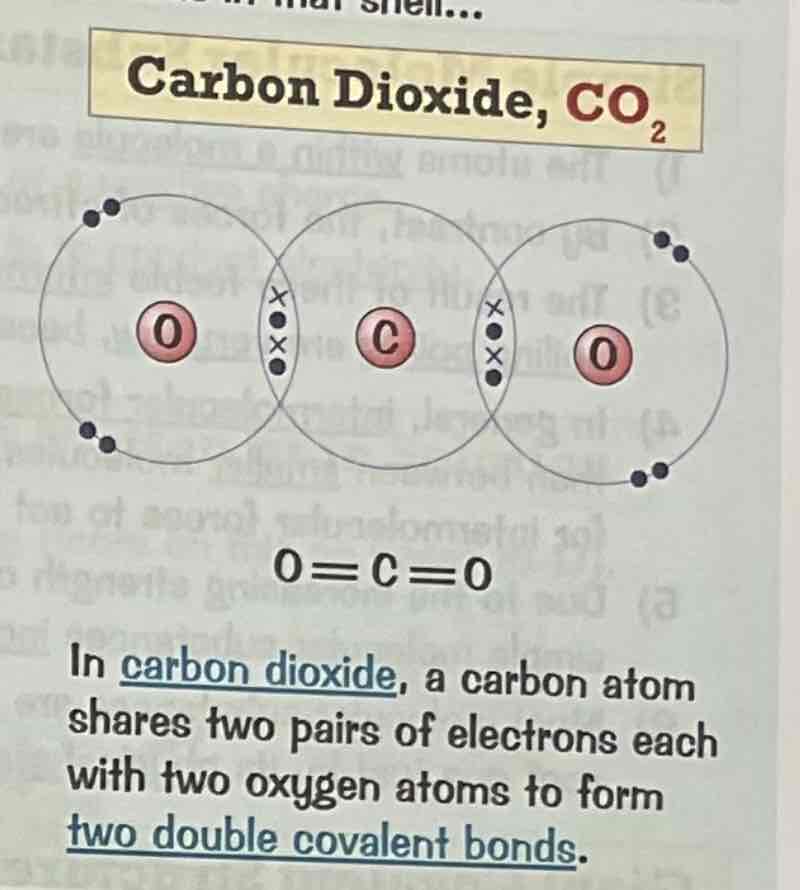
Draw a dot and cross diagram for the covalent bonding of methane, CH4
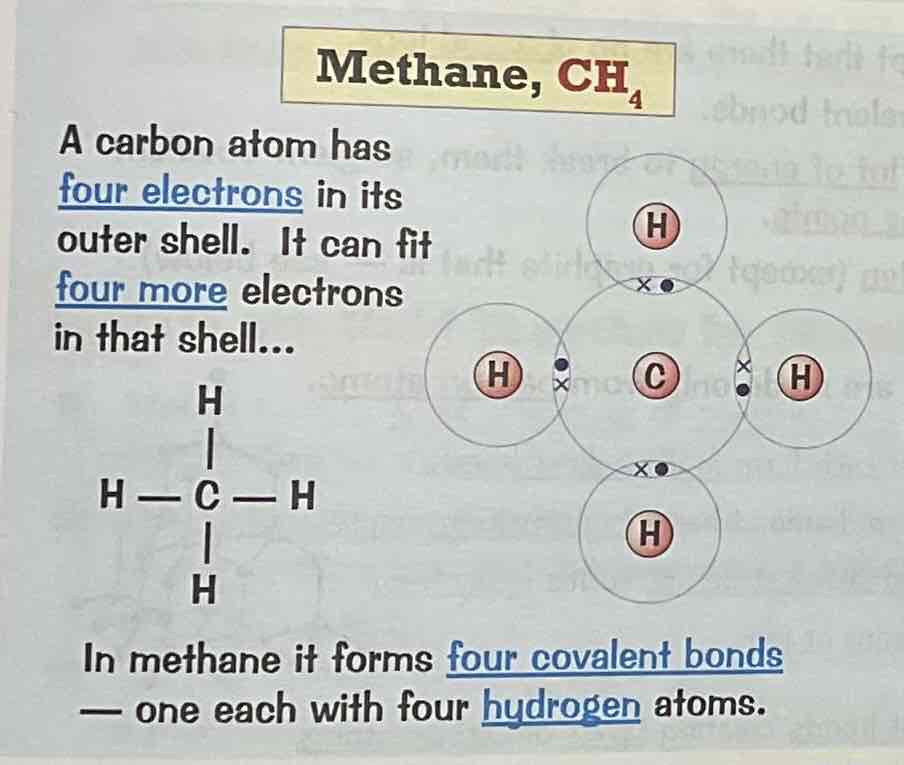
Draw a dot and cross diagram for the covalent bonding of ethane, C2H6
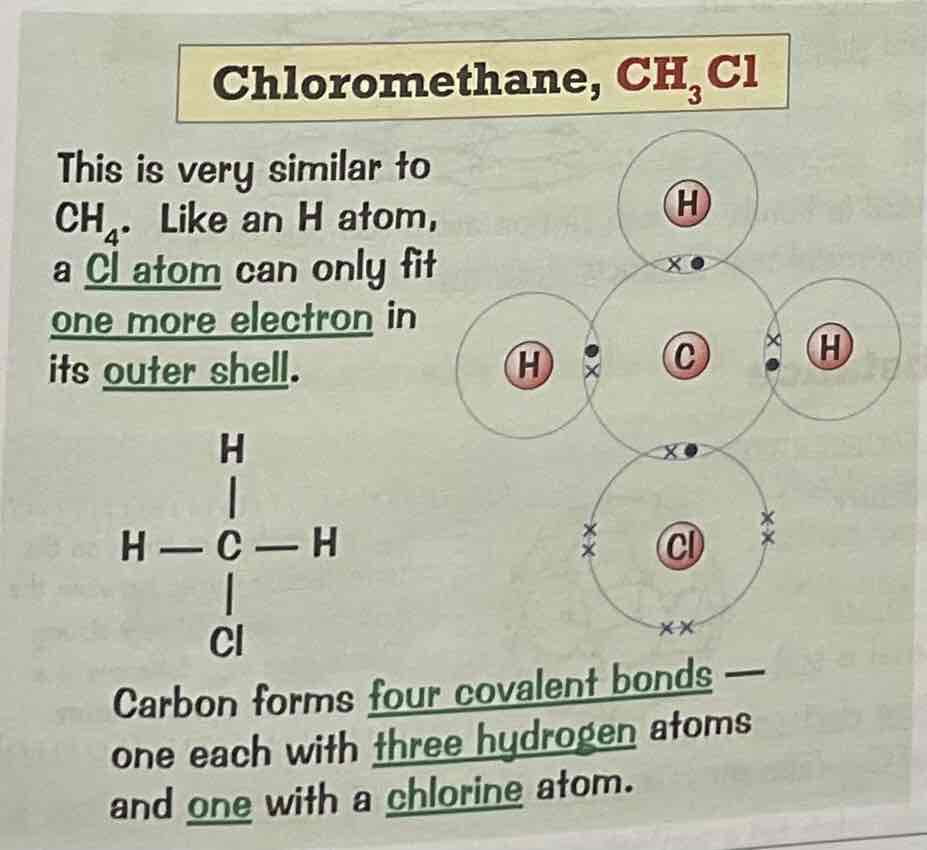
Draw a dot and cross diagram for the covalent bonding of Chloromethane, CH3Cl
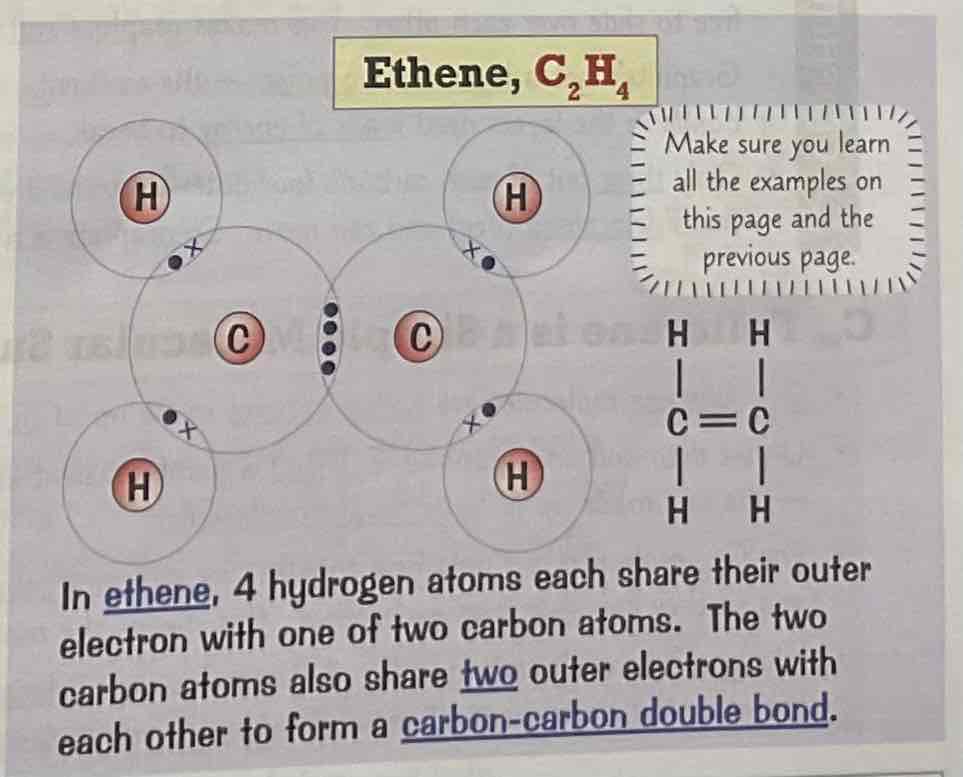
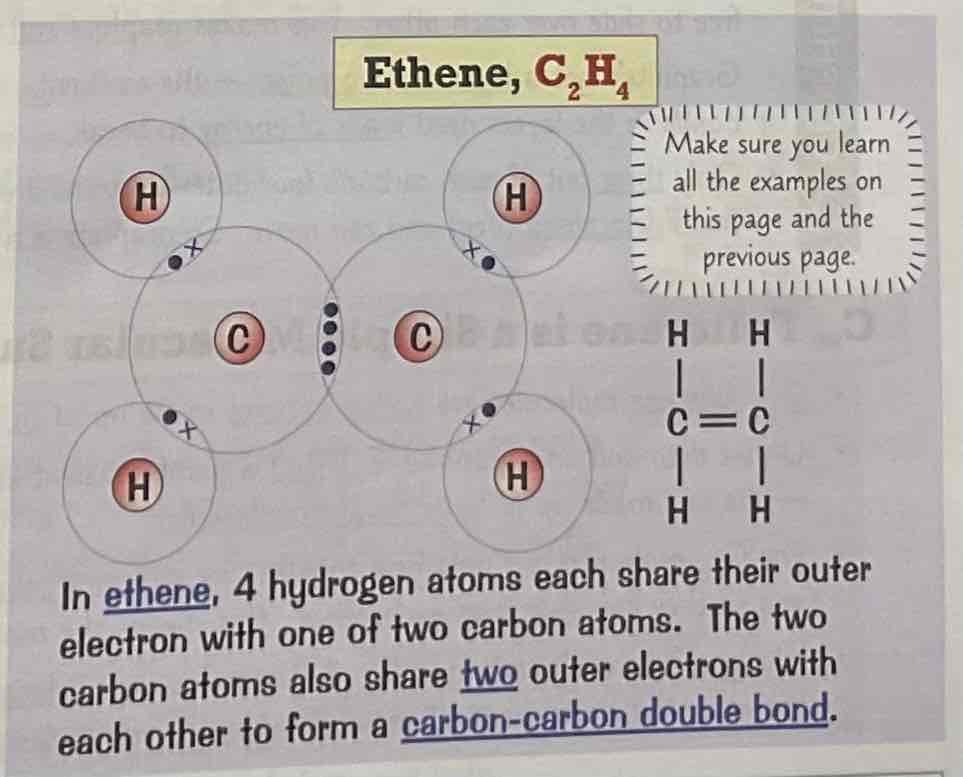
Draw a dot and cross diagram for the covalent bonding of ethane, C2H4
What can substances containing covalent bonds be? (Structures)
Simple molecules or giant structures
In simple molecular substances how are the atoms within a molecule held together?
Very strong covalent bonds
In simple molecular substances what are the forces of attraction between molecules like?
Very weak
In simple molecular substances how to the forces of attraction effect the melting and boiling points?
Weak intermolecular forces → melting and boiling points are very low, because the molecules are easily separated
What is the relationship between relative molecular mass (Mr) and size of molecule? Why?
In general, intermolecular forces are stronger between molecules with high relative molecular mass than between smaller molecules.
This is because there are more points along the larger molecules for intermolecular forces to act between them so more energy is needed to break the forces
How does melting + boiling point relate to Mr?
Due to the increasing strength of the forces, the melting and boiling points of simple molecular substances increase as the relative molecular mass increases.
What state are most molecular substances at room temperature?
Gas or liquid (or an easily melted solid)
What is the difference between giant covalent structures and giant ionic structures?
Giant ionic → there are no charged ions
What is the bond in giant covalent structures like
All the atoms are bonded to each other by strong covalent bonds
What is the melting/ boiling point of giant covalent structures like and why?
There are lots of strong covalent bonds which means it takes a lot of energy to break them → very high melting + boiling points
Do giant covalent structures conduct electricity?
No.
Expect graphite
Are giant covalent structures usually soluble in water?
No
What are two important examples and what are the made of?
Diamond and graphite
Only made from carbon atoms
Diamond: What is it made up of?
A network of carbon atoms that each form four covalent bonds
Diamond: melting point + why?
Strong covalent bonds take lots of energy to break → high melting point
Diamond: is it hard or soft + why?
The strong covalent bonds hold the atoms in a very rigid lattice structure so it’s really hard
Diamond: does it conduct electricity + why?
It doesn’t conduct electricity because it has no free electrons or ions
Graphite: what is it made up of?
Carbon atoms, each carbon atom only forms three covalent bonds, creating layers of carbon atoms