ACS FINAL
1/115
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
116 Terms
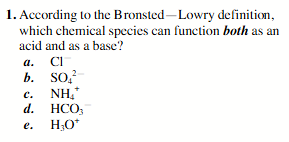
HCO3
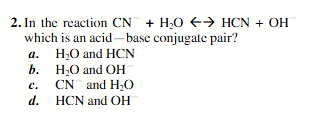
H2O and OH

It is less basic than a 1 M solution of NaY.

1.5
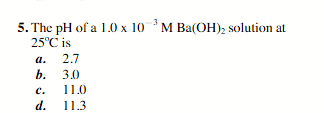
11.3
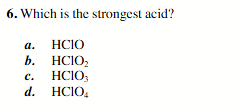
HClO4
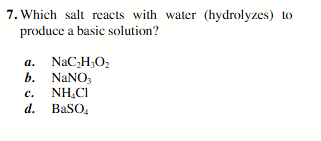
NaC2H3O2
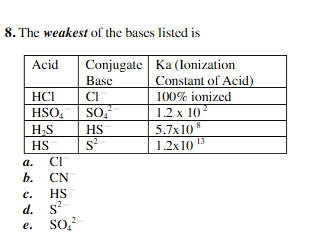
CI
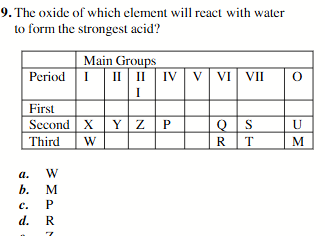
R
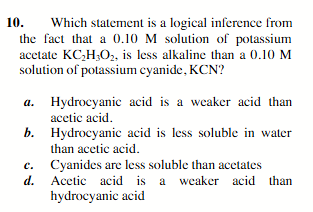
Hydrocyanic acid is a weaker acid than acetic acid.
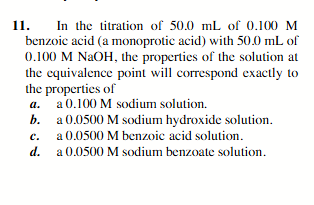
a 0.0500 M sodium benzoate solution.
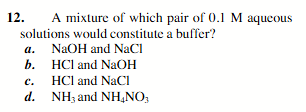
NH3 and HG4NO3
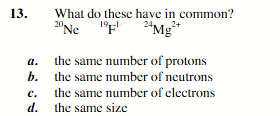
the same number of electrons

14

dumbbell shaped

5825p1

1s22s22p62d2

12.8
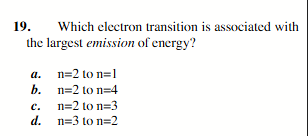
n=2 to n=1

energy is absorbed

lower energy
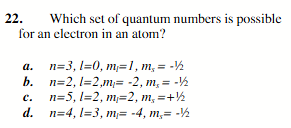
n=5, l=2, m1=2, ms=+1/2

ionic bonds
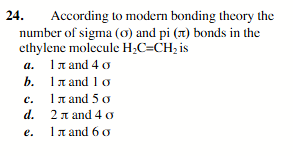
1 (pie) and 5 (sigma)
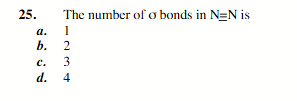
1

electrostatic forces of attraction

at which oxidation occurs

62
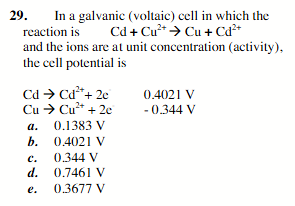
0.36777 V
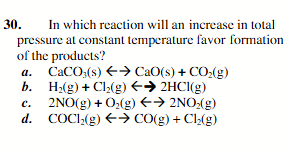
2NO(g)+ O2(g)←→2NO2(g)
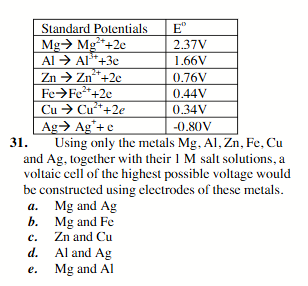
Mg and Ag
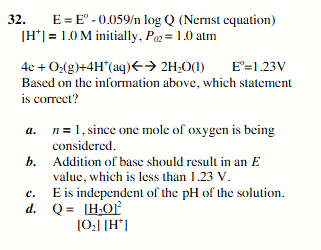
addition of base should result in an E value, which is less than 1.23V
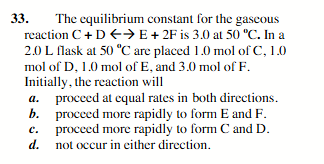
procced more rapidly to form C and D.
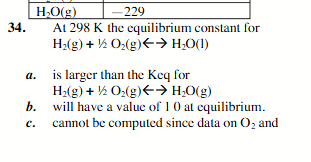
is larger than the keq for H2(g) +1/2 O2(g)←→H2O(g)
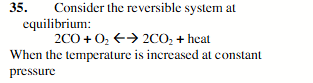
the CO2 concentration will be decreased
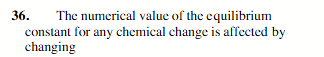
the temperature

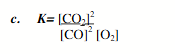

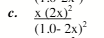

0.29
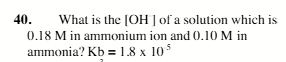
1.0×105
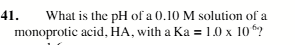
3.5
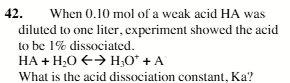
1 × 105
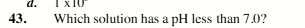
1 M NH4CI
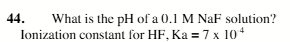
8.1
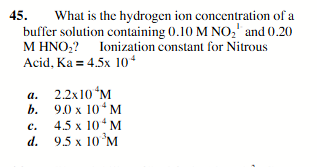
9.0 × 104M
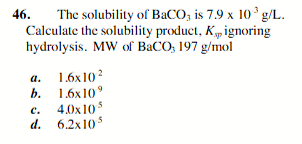
1.6 × 10 9

precipitation of more BaSo4
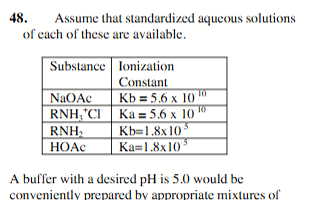
NaOAc and HOAc
C2H5OH
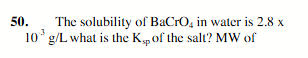
1.2 × 1010

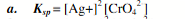
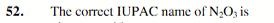
dinitrogen trioxide

FeCI3-iron(III) chloride
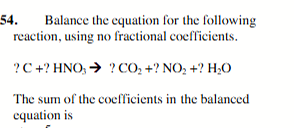
12

Ba3(PO4)2

gaseous molecules are continuously in random motion and collisions are perfectly elastic.

increasing the molecular concentration at constant temperature means increasing the number of collisions between molecules and container.

2/3 L
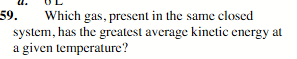
None: the average kinetic energy is the same for each gas

450 L

H2O
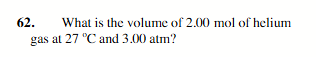
16.4 L
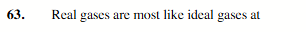
low pressure and high temperature.
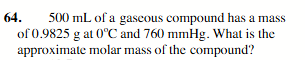
44.0
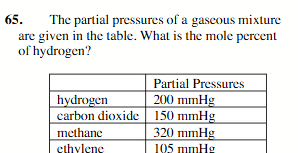
25.8

1 L(298/273)(760/(750-23.5)
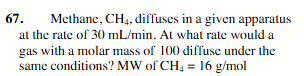
12.0 mL/min

decreases with increasing energy of activation.

be increased by a factor of 2.
provides an alternate path with a different activation energy.
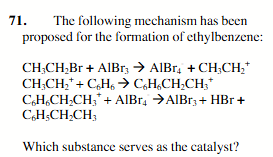
AIBr3

HF
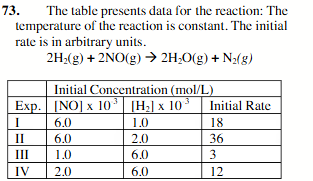
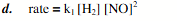

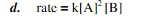

1.30g
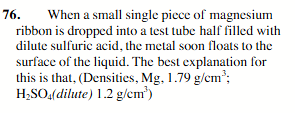
gas bubbles attached to the metal buoy the metal to the top.

the temperature only
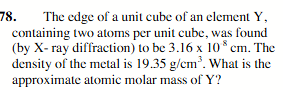
65.4
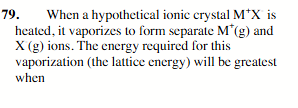
the effective radio of M+ and X+ are small

the higher the boiling point
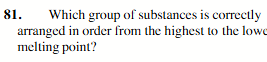
NaF>HF>H2
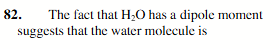
bent
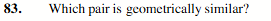
SO2 and O3
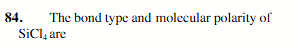
Polar and nonpolar

the nitrogen atom in NCI3 has a lone pair of electrons wheres the boron atom in BCLe does not.
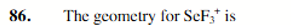
trigonal pyramidal
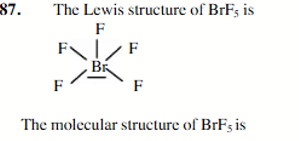
square pyramidal

CO3

tetrahedral

8 days

X-Y

the change of a neutron into a proton

C5H12

has a different structural formula
n-heptane C7H16
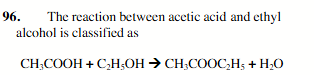
esterification
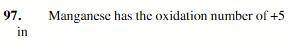
[Mn(CN)6]1
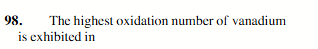
NH4VO3
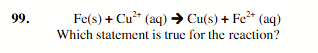
Cu2+ is reduced
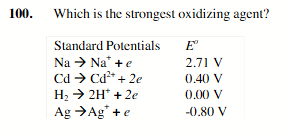
Ag+